Graphene–polyglycerol–curcumin hybrid as a near-infrared (NIR) laser stimuli-responsive system for chemo-photothermal cancer therapy†
Farhad Bania,
Mohsen Adeli*bc,
Soodabeh Movahedic and
Majid Sadeghizadeh*ad
aDepartment of Nanobiotechnology, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran. E-mail: sadeghma@modares.ac.ir
bInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Takustr, Berlin, Germany. E-mail: mohadeli@yahoo.com
cDepartment of Chemistry, Faculty of Science, Lorestan University, Khorram Abad, Iran
dDepartment of Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran
First published on 6th June 2016
Abstract
The aim of this study is to develop a nano graphene–polyglycerol–curcumin hybrid capable of simultaneous co-delivery of chemotherapeutic drug and cytotoxic heat to cancer cells by near infrared (NIR) laser irradiation. Based on a fluorescence quenching study, the nano graphene sheet was functionalized by a polyglycerol polymer via π–π interaction between the π conjugated system of graphene and the aromatic focal point of polyglycerol through static mechanism. The binding constant and standard Gibbs free energy change of the binding process, obtained from isothermal titration calorimetry (ITC), were 1.8 × 106 M−1 and −35.7 kJ mol−1, respectively. This hybrid showed excellent heat generation efficiency (ΔT ≈ 60 °C in 5 min, 1 W cm−1 NIR laser) and high loading capacity of curcumin (61%). The resulting drug delivery system displayed a good stability with low drug release in PBS. However, NIR laser irradiation could trigger release of curcumin from the surface of the functionalized graphene sheet in PBS (up to 5-fold greater release) due to the local generated heat. Also the laser induced intracellular release of curcumin was confirmed by flow cytometry analysis. So the combination of chemo/photothermal therapy using this advanced drug delivery system demonstrated a synergistic effect for killing of MCF-7 breast cancer cells by MTT and apoptosis assays.
Introduction
Since Geim and co-workers discovered graphene in 2004, it has been proposed for a wide range of applications due to its excellent electrical, mechanical, optical and thermal properties.1–3 Graphene is a single layer of sp2-hybridized carbon atoms arranged into a two-dimensional honeycomb lattice and is mostly obtained from graphite.4Macromolecules with hydrophobic blocks or π-conjugated systems are able to attach onto the surface or edges of graphene, owing to its high surface area and hydrophobic domains.5–7 This method, called noncovalent functionalization, is a well-known strategy to develop the functionality, solubility, processability and therefore intrinsic properties of graphene. The ability of graphene to form noncovalent interactions with other materials is a promising way to load and transport hydrophobic anticancer drugs to the target cells.8–11
Photothermal cancer therapies using graphene have also attracted significant research interest due to strong optical absorption of graphene in the near-infrared (NIR) region.12,13 The useful optical, mechanical, or electrical functions of graphene remain intact after noncovalent functionalization so noncovalent functionalization by using biocompatible and water soluble polymers, to increase half-life of graphene in the bloodstream for specific heating of tumor cells, is an important issue.14–17
There is a high interest in developing synergistic systems capable of simultaneous co-delivery of drug and photothermal agent which can enhance therapeutic efficiency with minimal side effects.18–20 Furthermore, a graphene sheet functionalized with biocompatible polymers and containing the loaded anticancer drugs could be a robust system to not only benefit synergic effects of photothermal therapy and chemotherapy but also control release of loaded drugs by NIR irradiation.21–23 There have been a few attempts at releasing doxorubicin from graphene based drug delivery systems induced by laser irradiation.24,25 But to the best of our knowledge, there is no similar report for curcumin.
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-eptadiene 3,5-dione), a polyphenol derived from the root of Curcuma longa, has demonstrated significant pharmacological activity including anti-inflammatory, antioxidant, analgesic, anticancer, etc.26 Curcumin can induce apoptosis in cancer cells without cytotoxic effects on healthy cells, therefore it is a potent natural agent for drug development against cancer.27 Despite its efficacy and safety, the pharmaceutical usefulness of curcumin is prevented by its poor aqueous solubility and bioavailability. It is particularly insoluble in water at acidic or neutral pH. At alkali pH, the dissociation of the acidic protons of curcumin raises its solubility, but due to the rapid hydrolytic degradation, it is not stable in this condition for a long time. In this regard, the application of nanocarriers represents a promising strategy to overcome the poor water solubility of curcumin and develop its bioavailability.28
In this work, noncovalent functionalization of nano graphene (nG) using hyperbranched polyglycerol with an aromatic focal point (PG) was developed. Then, the nG–PG hybrid with the loaded curcumin on its surface was used as a new system for combinational chemo-photothermal therapy and also to investigate the effect of NIR laser irradiation on the accelerated release of curcumin.
Experimental
Materials
Graphite, cyanuric chloride, H2SO4, H2O2, potassium permanganate (KMnO4), sodium nitrate (NaNO3), sodium hydride, p-phenylene diamine (PPD), acetone, tetrahydrofuran, dichloromethane (CH2Cl2), methanol, naphthol, diethanolamine and dimethyl formamide (DMF) were purchased from Merck Chemical Company. Glycidol, hydrazine (35 wt% in water) and curcumin were purchased from Sigma-Aldrich Company. A dialysis bag with a molecular cut-off 3.5 kDa was purchased from Sigma, USA.The MCF-7 human breast cancer cell line was obtained from the Pasteur Institute (Tehran, Iran). The high-glucose Dulbecco’s Modified Eagle’s Medium, fetal bovine serum, L-glutamine and penicillin/streptomycin, were all purchased from Thermo Fisher Scientific. The Annexin-V-FLUOS Staining kit was purchased from Roche Company.
Hyperbranched polyglycerol with naphthol rings at its focal point (PG) was synthesized according to the reported procedure in the literature.29
Characterization
FT-Infrared (FT-IR) experiments were performed using a PerkinElmer Inc. UV spectra were recorded on a Varian, Cary 100 spectrophotometer. Fluorescence measurements were performed on Varian, Carry Eclipse fluorescence spectrophotometer. Raman spectra were recorded using an Almega Thermo Nicolet Dispersive Raman Spectrometer. Ultrasonication was performed by a Hielscher, UP200H ultrasonic processor. The zeta potential and size measurement by the dynamic light scattering technique (DLS) of materials were performed using Brookhaven, PALS Zeta and 90plus analyzers. Surface imaging studies were performed using atomic force microscopy (AFM) to estimate surface morphology by a Veeco instrument. Isothermal titration calorimetry (ITC) experiments were performed using a VP-ITC Microcalorimeter, MicroCal at 25 °C. Transmission electron microscopy (TEM) analyses were performed by a Zeiss EM900 80 kV electron microscope. TEM samples were prepared by dropping a small quantity of dispersion onto carbon film on copper grids (300 mesh). The samples and the cells were irradiated with a laser model MDL-III-808 nm-2.5 W from Changchun New Industries Optoelectronics Tech. Company. The temperature of the dispersions was monitored using a digital thermometer with a thermocouple probe from the Pyrometer Instrument Company. Fluorescence images were taken with a Nikon TS100 invert Fluorescence microscope with a Hitachi camera. Flow cytometry tests were performed on a Partec space flow model flow cytometer using the FloMax software.Synthesis of nano graphene (nG)
Graphene oxide (GO) was synthesized with natural graphite powder using a modified Hummer’s method.30 To prepare nano-GO (nGO), the dried GO was redispersed in distilled water then was subjected to probe ultrasonication (50% amplitude, 400 W, 30 min) in an ice bath. Then the dispersion was centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 20 min) to remove precipitated un-exfoliated large GO sheets. The nGO sheets were freely dispersible in water without aggregation.
000 rpm, 20 min) to remove precipitated un-exfoliated large GO sheets. The nGO sheets were freely dispersible in water without aggregation.
For preparation of nG, the resulting homogeneous dispersion of nGO was added to dilute ammonia solution to adjust the solution to pH 10 and then added hydrazine monohydrate. The weight ratio of hydrazine to nGO was about 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.31 Then the solution was heated to 95 °C for 1 h. The excess hydrazine in the resulting dispersions was removed by dialysis against deionized water once the reduction was completed.
10.31 Then the solution was heated to 95 °C for 1 h. The excess hydrazine in the resulting dispersions was removed by dialysis against deionized water once the reduction was completed.
Study of interaction between nG and PG
| F = Finit × 10((Aex+Aem)/2) |
| F0/F = 1 + KSV[Q] |
Synthesis of nG–PG hybrid
nG–PG hybrid was prepared by mixing of nG dispersion in PBS buffer (10 ml, 0.2 mg ml−1) with 1 ml, 0.5 mg ml−1 PG polymer under ultrasonic oscillation. The resulting mixture was sonicated at room temperature for an additional 30 min and stirred overnight at room temperature to obtain the nG–PG hybrid. Also this method was applied for the preparation of nGO–PG. Other nG–PG hybrids were prepared by the same procedure with 0.1 and 1 mg ml−1 of PG solution.Protein adsorption
To determine protein adsorption, the mixture containing 0.2 mg ml−1 nG content of nG–PG hybrid and 0.2 mg ml−1 BSA protein was stirred with a magnetic stirrer for 24 h. The dispersed graphene particles were centrifuged and the concentration of BSA protein was determined in the supernatant by Bradford assay.32 This procedure was also applied for unfunctionalized nG.Photothermal effect of NIR laser irradiation on nG and nG–PG
nG and nG–PG dispersions in PBS with the same concentration of graphene content (20 μg ml−1) were irradiated with a 808 nm, continuous-wave NIR laser with a power density of 1 W cm−2 and the temperature of the samples was monitored using a digital thermometer with a thermocouple probe. Also this procedure was applied for nGO and nGO–PG dispersions.Loading of curcumin on nG–PG hybrid
Loading of curcumin onto nG–PG hybrids was accomplished by adding a curcumin solution in ethanol (0.5 ml, 1 mg ml−1) to aqueous dispersions of nG–PG hybrids (5 ml, 0.1 mg ml−1 nG content with different content of PG) under ultrasonic oscillation. Mixtures were stirred at room temperature overnight, then dispersions were dialyzed against water under light-proof conditions and remaining free curcumin was removed by centrifuge at 3000 rpm for 20 min. The loading capacity of curcumin on the functionalized graphene sheets was estimated by its absorption at 430 nm, after subtracting the absorption of functionalized graphene sheets based on the linear calibration curve using the curcumin solutions in ethanol with known concentrations. The curcumin loading capacity was calculated by the following equation:| Loading capacity = (amount of drug loaded/amount of graphene content of carrier) × 100 |
This procedure was also applied for loading of curcumin on nGO–PG hybrid.
Curcumin release study
The in vitro release of curcumin from nG–PG was performed by the dialysis method. nG–PG–Cur dispersion was placed in two dialysis bags with a molecular cut-off 12 kDa (2 ml equivalent to about 500 μg of curcumin). The dialysis bags were incubated in 20 ml of phosphate buffer solution (pH 7.4) containing Tween-80 (0.2% w/w) at 37 °C with gentle shaking. The release experiment was performed with and without NIR laser irradiation (5 min, 1 W cm−2) at predetermined interval times (at 0, 4, 8, 12, 24 and 36 times). The released curcumin was sampled at defined time period (before laser irradiation at predetermined interval times), freeze dried and then dissolved in ethanol for determination by UV-vis absorbance at 430 nm.Cell culture
MCF-7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM L-glutamine and 50 U ml−1 penicillin/streptomycin in a humidified incubator with 5.0% CO2 at 37 °C (complete DMEM media).Intracellular NIR-laser stimuli-responsive curcumin release
MCF-7 cells were seeded at 1 × 105 cells per well on glass cover slips in 6-well plates and incubated in a humidified 5% CO2 atmosphere at 37. When cells reached 70% confluence, they were treated with nG–PG–Cur and free curcumin (30 μM of curcumin content) for four hours followed by washing three times. The cells were observed by inverted fluorescence microscopy. The treated cells were trypsinized, washed and resuspended into DMEM media containing 10% FBS. 1 ml of each cell suspension was irradiated for 10 min in a 2 ml tube with an 808 nm continuous-wave NIR laser with a power density of 1 W cm−2 for 5 min, cells were washed and resuspended in PBS after irradiation. Then, the fluorescence intensity of cells was measured by flow cytometry.In vitro cytotoxicity study
For the cytotoxicity assay, MCF-7 cells were seeded in a 96-well plate at a density of 1 × 104 cells per well and incubated in 5% CO2 at 37 °C for 24 h prior to assay. The cells were exposed to different concentrations of free curcumin, nG–PG and nG–PG–Cur dispersed in the PBS buffer for 4 h. The ethanol percentage in the final culture medium for free curcumin was kept lower than 0.5% v/v. Cells were either irradiated or not irradiated with an 808 nm continuous-wave NIR laser with the power density of 1 W cm−2 for 2 min. Then, cells were incubated at 37 °C for an additional 48 h and finally cell viability was measured by using a standard MTT assay. The medium was replaced by fresh DMEM (100 μl) and then, MTT (10 μl, 5 mg ml−1) solution was added and incubated for 4 h. Finally, the medium was replaced with 100 ml of DMSO, and the absorbance was measured at 570 nm using a microplate reader. The relative cell viability was calculated according to the following equation:| Relative cell viability (%) = ((Atest − A0)/(Acontrol − A0)) × 100 |
Apoptosis assay
The cytotoxicity of chemo-photothermal treatment was also evaluated by quantification of apoptotic MCF-7 cells. MCF-7 cells were seeded at a density of 1 × 105 cells per well in 24-well plate and cultured overnight. The cells incubated with nG–PG–Cur hybrid with 30 μM concentration of the loaded curcumin for 4 h were either exposed or not exposed to 1 W cm−2 808 nm NIR laser irradiation for 2 min, and the cells were incubated for another 48 h. The cells were harvested, and the Annexin-V-FLUOS Staining kit was used to detect and quantify apoptosis by flow cytometry. Staining was performed according to the manufacturer’s instructions.Statistics
Statistical analysis was performed using the Student’s t test. A P value of less than 0.05 was considered as a statistically significant difference.Result and discussion
Graphene oxide (GO) was synthesized by the modified Hummers’ method,30 followed by strong sonication and centrifugation to remove the large GO layers and obtain nano-GO. Next, we reduced the nano-GO sheets by adding hydrazine monohydrate into the dispersion and heating to 95 °C for 60 min.The UV-vis spectra showed a red shift for the maximum absorbance (λmax) of GO from 228 to 258 nm upon reduction (Fig. S1†). In addition, the intensity of absorbance was significantly increased in the whole region to about 5.3 fold that in the NIR region centered on 808 nm. This result showed that the major part of the disrupted π conjugation system in graphene sheets has been restored by chemical reduction.26
The FT-IR spectra (Fig. S2†) confirmed the reduction of the oxygen-containing groups in nGO by hydrazine. Hydrazine did not remove all of the oxygen functional groups on the plane or edge of nGO, as already reported8,33 but clearly decreased the oxygen functional groups in nG.
The Raman spectra (Fig. S3†) of the nGO and nG showed that the ratio of D/G bands (at 1358 and 1597 cm−1, respectively) increased after reduction confirming that the reduction did take place.22
The as synthesized nG dispersion did not show a good colloidal stability in PBS and exhibited aggregation in one week. To improve the dispersion stability of graphene in aqueous solution, we easily functionalized nG noncovalently by a hyperbranched polyglycerol (PG) consisting of an aromatic segment as the focal point (Fig. 1). Polyglycerol, a biocompatible, protein resistant and water-soluble polymer which possesses a large number of functional groups is an alternative to PEG for biomedical applications.34–36
The aromatic segment of the PG polymer showed fluorescence emission with a peak centered at 350 nm at the excitation wavelength of 215 nm (Fig. 2a). In order to study interactions between PG and nG, changes in the intrinsic fluorescence of the aromatic focal point of polymer were evaluated during titration with nG. The intensity of fluorescence of the PG solution gradually decreased with increasing nG concentration in the mixture. This observation indicated that the aromatic focal point of PG has interacted with the π conjugated domains of the nG sheet via π–π stacking interactions leading to graphene-induced fluorescence quenching (Fig. 2a). Also, in order to understand the mechanism of the fluorescence quenching, the interaction between the polymer and nG was evaluated by fluorescence spectroscopy at three different temperatures. In general quenching can either occur dynamically or statically showing a different dependence on temperature.37 Dynamic quenching is caused by diffusion, therefore higher temperatures result in larger diffusion coefficients and the extent of dynamic quenching depends on temperature directly. In contrast, static quenching is caused by ground-state complex formation. Hence, higher temperatures will usually result in decreased stability of complexes, and thus lower static quenching. The plots of F0/F versus [Q] were found to be linear at three different temperatures (Fig. 2b) suggesting that the π conjugated domains of the nG sheet were equally accessible to the aromatic focal point of PG.38 The slope of the Stern–Volmer plot was decreased with increasing temperature (Fig. 2b) indicating that the PG bound to the nG sheet by ground-state complex formation inducing the static fluorescence quenching mechanism.
Since the slope of the Stern–Volmer plot for titration of PG with nG at 25 °C was larger than that for nGO (Fig. S4b†), it is concluded that the nG is a more efficient fluorescence quencher as compared with nGO as already reported by florescence quenching microscopy.39 The quenching mechanism could be explained by photo-induced electron transfer between the aromatic molecules and graphene.40 In this mechanism, the energy level of graphene is lower than graphene oxide, therefore the electron transfer from the excited aromatic dye molecules to rGO is easier than to GO.
The binding affinity between nG and PG was determined by an ITC experiment which showed an exothermic signal during titration of nG with PG at 25 °C as shown in Fig. 3. According to analysis of the ITC data, PG could bind to the nG sheet with a favorable binding constant (Kb = 1.8 × 106 M−1). The enthalpy (ΔH = −22 kJ mol−1) contributed more to the standard Gibbs free energy change (ΔG = −35.7 kJ mol−1) in comparison with entropy (TΔS = 13.7 kJ mol−1) during the hybridization process which means the noncovalent interaction is the driving force for binding. The number of binding sites, n, on the surface of nG to PG was equal to 100.
 | ||
| Fig. 3 Titration of nG by PG at 25 °C, showing heat flow as a function of time and integrated heat vs. the molar ratio (enthalpogram). | ||
As the ITC result showed, the surfaces of the nG sheets were saturated by PG polymer at a PG/nG molar ratio of about 200. So we used this ratio as an optimum ratio to produce nG–PG hybrid. However in order to investigate the effect of amount of PG on the stability of dispersion and drug loading capacity of the hybrid, we also used a frame of different concentration of PG to produce nG–PG hybrids. The nG–PG hybrid with a low level of PG exhibited aggregation in one week but the nG–PG hybrids with optimum and high level of PG were stable in PBS and no precipitation was observed even after several weeks. We selected the nG–PG with an optimum level of PG for further analysis.
In the FT-IR spectra of the nG–PG hybrid (Fig. S2†), increasing the intensity of absorbance bands of hydroxyl functional groups at around 3400 and also aliphatic C–H groups at 2877 and 2926 cm−1 together with the appearance of an intense absorbance band at 1074 and 1103 cm−1 for C–O bonds confirmed functionalization of nG by PG polymer. As can be seen in Fig. S2,† the absorbance bands for aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of both nG and PG and also C
C bonds of both nG and PG and also C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds of PG, at 1600–1700 cm−1 region, changed substantially in the nG–PG hybrid and they appeared as a new single band at 1622 cm−1. This result proved that noncovalent interactions between nG and PG are dominated by aromatic rings of both systems.
N bonds of PG, at 1600–1700 cm−1 region, changed substantially in the nG–PG hybrid and they appeared as a new single band at 1622 cm−1. This result proved that noncovalent interactions between nG and PG are dominated by aromatic rings of both systems.
Zeta potential analyses showed a high negative surface charge for the nGO sheet (−51 mV) in PBS, due to ionization of the carboxylic acid groups. The surface charge of nGO in PBS was diminished to −25 mV upon reduction because of the decreasing number of ionizable groups. Also, the zeta potential of the nG sheet in PBS was diminished to −11 mV after functionalization by PG, confirming that the nG sheet was functionalized by PG.
The morphology and size of the nG were investigated before and after functionalization with PG by AFM (Fig. 4). According to AFM images, the thickness of the nG was increased from 1.2 nm to 5.6 nm after functionalization by PG (Fig. 4) assigned to the attachment of polymer to monolayer nG sheets. This finding was confirmed by the increase of the mean diameter of nG, measured by the DLS technique after functionalization with PG (Fig. S5†). The mean diameter of nG and nG–PG were 50, 65 nm respectively. Comparing the TEM images of nG and nG–PG hybrid clearly showed the attachment of the PG polymer to the surface of the nG sheets changing the contrast and morphology of the nG sheets (Fig. 5).
A BSA protein adsorption of 13 mg per gram of nG was obtained for PG functionalized nG while the unfunctionalized nG adsorbed 280 mg of protein per gram of nG. This finding indicated that polyglycerol branches located at the nG surface not only stabilized nG aqueous dispersion but also improved resistance of nG to the nonspecific interactions with plasma proteins. It could be also found that a noncovalent interaction between PG and nG was strong enough to stay in the associate form even in the presence of proteins.
The photothermal property of dispersions of nGO, nGO–PG, nG, nG–PG in PBS was evaluated using NIR laser irradiation (808 nm, 1 W cm−2). In contrast to PBS solution which was used as a blank dispersion, significant increases in the temperature of dispersions were seen after 5 min irradiation (Fig. 6). The heat generation efficiencies of nG–PG and nG with the same nG content (20 μg ml−1) were very close to each other (ΔT ≈ 60 °C in 5 min). Also, the same result was found for nGO–PG and nGO dispersions (ΔT ≈ 38 °C in 5 min). This finding indicates that noncovalent attachment of PG polymer to graphene sheets did not affect the structure and photothermal property of graphene. In addition, the heat generation efficiency of nG–PG was significantly higher than nG–PG due to the healing of the conjugated aromatic system of graphene and efficient absorption of NIR light by it (Fig. 1S†).
Recording of the temperature elevation of nG–PG and nGO–PG dispersions over five NIR laser irradiation on/off cycles showed that in contrast to the Au nanorods as well-known photothermal agents,41 the absorption spectra (Fig. S6a†) and the photothermal property (data not shown) of these hybrids did not change after five on/off cycles because of their excellent photostability during the temperature elevation under laser irradiation. Also, the intrinsic florescence intensity of PG polymer did not increase after five laser irradiation on/off cycles confirming that there was not significant detachment of PG polymer from nG and nGO sheets during the temperature elevation (Fig. S6b†).
Curcumin was easily loaded on the nG–PG hybrid by a simple mixing of an ethanol solution of curcumin and an aqueous dispersion of nG–PG. Curcumin has extensive π–π conjugation with two aromatic moieties that induce hydrophobic interactions and π–π stacking onto the surface of graphene sheets. The broadening of the curcumin absorbance peak at 430 nm after loading on nG–PG indicated that there were interactions between the π conjugated systems of graphene sheet and curcumin (Fig. S1†). The successful adsorption of drug onto the surface of a graphene sheet of the nG–PG hybrid led to intensive fluorescence quenching of the drug (Fig. S7†).
The loading capacity of curcumin on the functionalized graphene sheets was estimated by its absorption at 430 nm, after subtracting the absorption of carrier (Fig. S1†) using the calibration curve (Fig. S8†). As can be seen in Fig. S1,† the nG–PG hybrid exhibited a higher loading capacity of curcumin (about 61%) than that of the nGO–PG hybrid (about 19%). The less disrupted π conjugation system in nG led to better interaction between the functionalized graphene sheets and curcumin so the loading capacity of nG–PG was higher than the nGO–PG hybrid. Also we found that the nG–PG hybrid with a low level of PG exhibited a higher loading capacity of curcumin (about 73%) in comparison with the nG–PG hybrid with an optimum level of PG (about 61%) due to the higher unoccupied surface of nG by PG but this drug hybrid did not have good dispersion stability. The nG–PG hybrid with a high level of PG showed about the same loading capacity of curcumin (about 59%) in comparison with the nG–PG hybrid with an optimum level of PG indicating that the unbound PG polymer did not affect the curcumin loading.
We did not find a significant change in zeta potential of nG–PG after curcumin loading (−12 mV) suggesting that the branches of PG have covered the surface of graphene even after loading of curcumin on the graphene surface. Also the hydrodynamic mean diameter (Fig. S5†) and height (Fig. 4) of the nG–PG hybrid was increased to 73 and 7.1 nm respectively by loading curcumin on it. TEM images show that after interactions between graphene and polymer the contrast as well as morphology has changed. However, there are no significant changes in the morphology of nG–PG, after loading the drug (Fig. 5).
The nG–PG–Cur drug delivery system was expected to release the loaded drug controllably by NIR laser irradiation. This anticancer drug delivery system exhibited an excellent stability in the physiological medium with about 6% release over 48 h. The cumulative release of curcumin from the drug hybrid reached 29% by 5 min laser irradiation of the drug hybrid dispersion at predetermined interval times for a total of 30 min of laser irradiation over 48 h (Fig. 7). This 5-fold greater release of curcumin from the drug hybrid by laser irradiation in comparison to that without irradiation suggested the heat-stimulated dissociation of curcumin from the hybrid. The local heat generated from the surface of graphene sheet by absorbing laser energy, as shown in Fig. 6, increased the molecular movement of curcumin on the surface of graphene. This increase was high enough to weaken the noncovalent interaction between curcumin and the graphene sheet resulting in higher cumulative release of drug from the hybrid.
The efficiency of the drug delivery system to transfer curcumin to the cells was evaluated by using the intrinsic green fluorescence of curcumin. The observed green fluorescence inside the MCF-7 cells treated with curcumin and nG–PG–Cur hybrid, proved that curcumin was able to enter the cancer cells along with nG–PG–Cur hybrid (Fig. 8).
 | ||
| Fig. 8 Visible light (left) and fluorescence (right) images of (a) control MCF-7 cells, MCF-7 cells treated with (b) nG–PG–Cur and (c) free curcumin (solublized with 1% ethanol) for 4 h. | ||
The fluorescence profile of the cells treated with nG–PG–Cur showed a distinct right shift after laser irradiation while laser irradiation did not affect the fluorescence signal of curcumin in the cells treated with free curcumin (Fig. 9). The cells treated with nG–PG–Cur exhibited a 4-fold higher mean fluorescence intensity after 10 min laser irradiation. The fluorescence of curcumin loaded on nG–PG was initially quenched by the graphene sheet. Upon laser irradiation of the cells, the photothermal induced desorption of curcumin from the surface of graphene resulted in the recovery of curcumin fluorescence, so the fluorescence intensity of the cells was increased. The accelerated release of doxorubicin from graphene carrier by laser irradiation has recently been reported.24 Also this finding showed that curcumin could enter into MCF-7 cells at a higher amount by nG–PG hybrid in comparison with free curcumin.
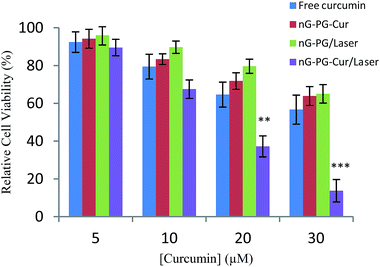
The cytotoxicity of nG–PG–Cur toward MCF-7 breast cancer cells was investigated by standard MTT assays. nG–PG showed no significant cytotoxicity after 48 h incubation even in concentrations as high as 100 μg ml−1 (<10%) indicating the biocompatible nature of the drug carrier (Fig. S9†). nG–PG–Cur exhibited approximately similar in vitro anticancer efficacy to that exhibited by free curcumin (Fig. 10). However, one of the most important limitations with curcumin is rapid metabolism of curcumin in vivo and loading of curcumin onto nG–PG hybrid may result higher drug bioavailability and anticancer efficacy.
To estimate the effect of NIR laser irradiation on the anticancer efficiency of nG–PG–Cur hybrid, we evaluated photothermal therapy by nG–PG carrier and combined chemo-photothermal therapy by nG–PG–Cur hybrid along with chemotherapy. The cells were treated with nG–PG/laser irradiation, nG–PG–Cur/laser irradiation, nG–PG–Cur and free curcumin at different concentrations. Carrier and drug contents were fixed at the same level for all systems. The NIR laser irradiation did not affect the control cell viability and also did not induce higher cytotoxicity for free curcumin (data not shown). The carrier showed cytotoxicity under NIR laser irradiation because of the photothermal effect of graphene (Fig. 10). For the nG–PG–Cur hybrid with 30 μM concentration of loaded curcumin, the inhibition rate of chemo-photothermal therapy (nG–PG–Cur/laser) was significantly increased to 86%, while this parameter for chemotherapy (nG–PG–Cur) and photothermal therapy (nG–PG/laser) was 35% and 36% respectively (Fig. 10). This result indicated that the combination of chemotherapy and phototherapy exhibits a synergistic effect due to accelerated curcumin release from the graphene hybrid by NIR-laser irradiation.
Also, the effect of laser irradiation on the cytotoxicity of the nG–PG–Cur hybrid was also evaluated by quantification of apoptotic MCF-7 cells. The cells were treated with nG–PG–Cur hybrid with 30 μM concentration of the loaded curcumin and with or without laser irradiation, and then stained with PI and Annexin V. Annexin V-positive, PI-negative cells were considered as apoptotic. Double-stained cells were considered as necrotic/late apoptotic cells. As shown in Fig. 11, the nG–PG–Cur hybrid plus laser significantly induced cell apoptosis and late apoptosis/necrosis (71.9%) compared with nG–PG–Cur alone (36.6%). This result indicated that the cytotoxic effect of nG–PG–Cur hybrid on MCF-7 cells could be significantly improved by the laser irradiation.
 | ||
| Fig. 11 Apoptosis assay of (a) control MCF-7 cells, MCF-7 cells treated with (b) nG–PG–Cur and (c) nG–PG–Cur/laser. | ||
Conclusion
The nG–PG hybrid prepared by mixing nG dispersion with PG solution exhibited excellent aqueous colloidal stability and heat generation efficiency under NIR laser irradiation. It was observed that NIR laser irradiation could accelerate release of curcumin from the curcumin loaded nG–PG hybrid due to the heat-stimulated dissociation of curcumin from the surface of the functionalized graphene sheet. Therefore, the combination of photothermal and chemotherapy by using the nG–PG–Cur advanced drug delivery system exhibited a synergistic effect for killing the cancer cells.References
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- Y. Zhang, T. R. Nayak, H. Hong and W. Cai, Nanoscale, 2012, 4, 3833–3842 RSC.
- Z. Fan, S. Li, F. Yuan and L. Fan, RSC Adv., 2015, 5, 19773–19789 RSC.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- J. Liu, L. Tao, W. Yang, D. Li, C. Boyer, R. Wuhrer, F. Braet and T. P. Davis, Langmuir, 2010, 26, 10068–10075 CrossRef CAS PubMed.
- C.-W. Liu, F. Xiong, H.-Z. Jia, X.-L. Wang, H. Cheng, Y.-H. Sun, X.-Z. Zhang, R.-X. Zhuo and J. Feng, Biomacromolecules, 2013, 14, 358–366 CrossRef CAS PubMed.
- S. S. Banerjee, A. Jalota-Badhwar, K. R. Zope, K. J. Todkar, R. R. Mascarenhas, G. P. Chate, G. V. Khutale, A. Bharde, M. Calderon and J. J. Khandare, Nanoscale, 2015, 7, 8684–8688 RSC.
- X. T. Zheng and C. M. Li, Mol. Pharm., 2012, 9, 615–621 CrossRef CAS PubMed.
- F. M. Tonelli, V. A. Goulart, K. N. Gomes, M. S. Ladeira, A. K. Santos, E. Lorençon, L. O. Ladeira and R. R. Resende, Nanomedicine, 2015, 10, 2423–2450 CrossRef CAS PubMed.
- M. Xie, H. Lei, Y. Zhang, Y. Xu, S. Shen, Y. Ge, H. Li and J. Xie, RSC Adv., 2016, 6, 9328–9337 RSC.
- S. Some, A.-R. Gwon, E. Hwang, G.-h. Bahn, Y. Yoon, Y. Kim, S.-H. Kim, S. Bak, J. Yang and D.-G. Jo, Sci. Rep., 2014, 4, 6314 CrossRef CAS PubMed.
- M. Rahman, S. Akhter, M. Z. Ahmad, J. Ahmad, R. T. Addo, F. J. Ahmad and C. Pichon, Nanomedicine, 2015, 10, 2405–2422 CrossRef CAS PubMed.
- M. J. Hajipour, O. Akhavan, A. Meidanchi, S. Laurent and M. Mahmoudi, RSC Adv., 2014, 4, 62557–62565 RSC.
- W. Miao, G. Shim, S. Lee and Y.-K. Oh, Biomaterials, 2014, 35, 4058–4065 CrossRef CAS PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, D. Vinh and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- O. Akhavan, E. Ghaderi, S. Aghayee, Y. Fereydooni and A. Talebi, J. Mater. Chem., 2012, 22, 13773–13781 RSC.
- D. K. Lim, A. Barhoumi, R. G. Wylie, G. Reznor, R. S. Langer and D. S. Kohane, Nano Lett., 2013, 13, 4075–4079 CrossRef CAS PubMed.
- J. Shi, L. Wang, J. Zhang, R. Ma, J. Gao, Y. Liu, C. Zhang and Z. Zhang, Biomaterials, 2014, 35, 5847–5861 CrossRef CAS PubMed.
- H.-W. Yang, Y.-J. Lu, K.-J. Lin, S.-C. Hsu, C.-Y. Huang, S.-H. She, H.-L. Liu, C.-W. Lin, M.-C. Xiao, S.-P. Wey, P.-Y. Chen, T.-C. Yen, K.-C. Wei and C.-C. M. Ma, Biomaterials, 2013, 34, 7204–7214 CrossRef CAS PubMed.
- Y. W. Chen, P. J. Chen, S. H. Hu, I. W. Chen and S. Y. Chen, Adv. Funct. Mater., 2014, 24, 451–459 CrossRef CAS.
- S.-H. Hu, R.-H. Fang, Y.-W. Chen, B.-J. Liao, I. W. Chen and S.-Y. Chen, Adv. Funct. Mater., 2014, 24, 4144–4155 CrossRef CAS.
- J. Bai, Y. Liu and X. Jiang, Biomaterials, 2014, 35, 5805–5813 CrossRef CAS PubMed.
- X. Shi, H. Gong, Y. Li, C. Wang, L. Cheng and Z. Liu, Biomaterials, 2013, 34, 4786–4793 CrossRef CAS PubMed.
- H. Kim, D. Lee, J. Kim, T.-i. Kim and W. J. Kim, ACS Nano, 2013, 7, 6735–6746 CrossRef CAS PubMed.
- C. Xu, D. Yang, L. Mei, Q. Li, H. Zhu and T. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12911–12920 Search PubMed.
- H. Zhou, C. S. Beevers and S. Huang, Curr. Drug Targets, 2011, 12, 332 CrossRef CAS PubMed.
- M. López-Lázaro, Mol. Nutr. Food Res., 2008, 52, S103–S127 Search PubMed.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Drug Discovery Today, 2012, 17, 71–80 CrossRef CAS PubMed.
- S. Movahedi, M. Adeli, A. K. Fard, M. Maleki, M. Sadeghizadeh and F. Bani, Polymer, 2013, 54, 2917–2925 CrossRef CAS.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137–3140 CrossRef CAS PubMed.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- H. Kim and W. J. Kim, Small, 2014, 10, 117–126 CrossRef CAS PubMed.
- M. Calderón, M. A. Quadir, S. K. Sharma and R. Haag, Adv. Mater., 2010, 22, 190–218 CrossRef PubMed.
- J. Khandare and M. Calderón, Nanoscale, 2015, 7, 3806–3807 RSC.
- M. Adeli, H. Namazi, F. Du, S. Hönzke, S. Hedtrich, J. Keilitz and R. Haag, RSC Adv., 2015, 5, 14958–14966 RSC.
- F. Faridbod, M. R. Ganjali, B. Larijani, S. Riahi, A. A. Saboury, M. Hosseini, P. Norouzi and C. Pillip, Spectrochim. Acta, Part A, 2011, 78, 96–101 CrossRef PubMed.
- L. Peng, H. Minbo, C. Fang, L. Xi and Z. Chaocan, Protein Pept. Lett., 2008, 15, 360–364 CrossRef CAS PubMed.
- J. Kim, L. J. Cote, F. Kim and J. Huang, J. Am. Chem. Soc., 2009, 132, 260–267 CrossRef PubMed.
- Y. Liu, C.-y. Liu and Y. Liu, Appl. Surf. Sci., 2011, 257, 5513–5518 CrossRef CAS.
- G. Fu, W. Liu, S. Feng and X. Yue, Chem. Commun., 2012, 48, 11567–11569 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05917a |
| This journal is © The Royal Society of Chemistry 2016 |