Emergence of fluorescence in boron nitride nanoflakes and its application in bioimaging†
Abstract
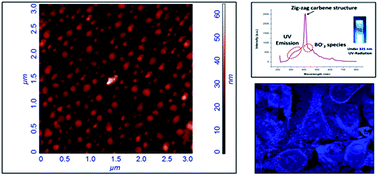
Hexagonal boron nitride (h-BN) has a layered structure similar to graphite and can be synthesized in the forms of nanotubes and nanosheets. These forms of BN have potential applications in mechanical, structural, optoelectronics and semiconductor devices. The formation of quantum dots of BN has expanded new physical properties such as fluorescent behavior. In the present study, hexagonal boron nitride (h-BN) nanoflakes have been synthesized by reaction of B2O3 and (NH2)2CO under a controlled atmosphere. BN nanoflakes, as observed in transmission electron microscopy (TEM) and scanning electron microscopy (SEM), were found to have wide size ranges of 6–35 nm. HRTEM for a single particle was also performed to confirm the formation and interplanar spacing corresponds to h-BN. Fourier transformation infrared spectroscopy (FTIR) spectra indicate strong B–N absorption at 1410 cm−1 and 799 cm−1. Absorption spectra of the product, investigated by UV-Vis spectrophotometry, reveal the characteristic peak of h-BN at 205 nm. Luminescence was recorded by fluorescence spectrophotometry and strong luminescence peaks at 411 nm and 435 nm, both in the blue region of the visible spectra, were found. Fluorescence properties of BN nanoflakes were used for imaging of cancer cells at different concentrations. BNNFs were found to be biocompatible against breast cancer celline MCF-7.

 Please wait while we load your content...
Please wait while we load your content...