Morphology engineering: dramatic roles of serine and threonine in supramolecular assembly†
Abstract
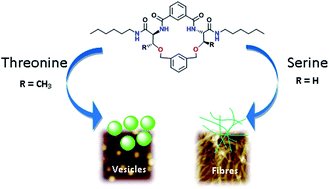
A series of lipidated macrocycles M1–M4 based on serine and threonine was designed and synthesized. Interestingly, threonine containing macrocycles assemble to vesicles, while serine-based macrocycles prefer to form fibrillar assemblies. Serine macrocycles with a 1,3-benzene dicarbonyl spacer forms fibrils while those with a biphenyl spacer resulted in a morphological change to vesicles.

 Please wait while we load your content...
Please wait while we load your content...