Y2O3 functionalized natural palygorskite as an adsorbent for methyl blue removal
Xi He†
ab,
Jianjun Wang†ab,
Zhan Shuab,
Aidong Tang*c and
Huaming Yang*ab
aCentre for Mineral Materials, School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China. E-mail: hmyang@csu.edu.cn; Fax: +86 731 88830549; Tel: +86 731 88830549
bKey Laboratory for Mineral Materials and Application of Hunan Province, Central South University, Changsha 410083, China
cSchool of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: adtang@csu.edu.cn; Fax: +86 731 88879616; Tel: +86 731 88879616
First published on 20th April 2016
Abstract
Y2O3 functioned palygorskite (Pal) composite as a novel adsorbent has been successfully synthesized and characterized, which shows stable and rapid decolorization performance for methyl blue (MB). HRTEM images showed that Y2O3 nanoparticles with size about 2–5 nm evenly dispersed on palygorskite, and the increase of the binding energy of Y2O3 (Y3d5/2) confirmed that several bonds such as Y–OH and Y–O–Si were existed in Y2O3/Pal adsorbent. Y2O3 modification greatly increased the number of negatively charged groups as Y2O3/Pal showed lower negative zeta potential than that of Pal. Therefore, the electrostatic interaction between Y2O3/Pal and MB is impossible to be the adsorption mechanism. What's more, it is found that the adsorption isotherm obeys the Langmuir model, with the maximum adsorption capacity greatly enhanced to 1579.06 mg g−1, exhibiting potential applications in wastewater treatment.
1. Introduction
The dyes are generally used in leather, textile, plastics, food and paper industries. They are difficult to decompose because of their complex components, dark color and high levels of organic pollutants. The methods commonly adopted to decompose organic dye wastewater include chemical oxidation,1–3 biological treatment,4–6 and photocatalytic degradation.7–9 However, the processes mentioned above are limited to a certain extent. Chemical oxidation shows high efficiency and high cost. Biological process is not applicable because organic dye wastewater contains toxic or non-biodegradable substances. Photocatalytic degradation needs UV irradiation. Although the emerging ozonation method is quite effective in decolorizing dye wastewater, it would lead to additional problems such as high pH values.10Adsorption has attracted great attention because it can operate effectively without UV irradiation and oxidant. The latest finds suggest that the adsorption performance of a adsorbent could be substantially enhanced by forming hydrogen bond with dye molecule,11–15 in which the rules of design of composites that could decolorize dye wastewater in an efficient and rapid way are suggested. Palygorskite is a natural product with large specific surface area and adsorption capacity.16–18 Palygoskite is very cheap and offers an option for the removal of organic and inorganic contaminants.19–24 Palygorskite is readily available materials functioning as excellent cation exchangers, which have often been used to adsorb metallic contaminants.25–27
Y2O3 is a promising host matrix for luminescence due to its good chemical durability, thermal stability.28 Y2O3 can host optically active dopants, stabilize metal oxides for using in catalytic and ceramic materials.29–31 The potential application of Y2O3 in dye wastewater purification is suggested since it could react with water to produce YOOH,32 while YOOH might greatly accelerate the decolorization by forming bond with dye molecule. However, to the best of our knowledge, Y2O3 has not been applied in the purification of dye wastewater.
Our group has done a lot of research on surface functionalization33–46 and structure modification47–52 of clay minerals, in which the surface or structure properties of clay minerals can be adjust according to the demand. Y2O3/Pal might be an option for the rapid purification of dye wastewater as both of them have special surface properties. Meanwhile, the safety assessment for Y2O3/Pal composite is also very important. According to Yttrium oxide (Y2O3) Material Safety Data Sheet (MSDS),53 Y2O3 is a white and odourless powder. Its water solubility is insoluble. Toxicity is LD50 intraperitoneal – rat – 230 mg kg−1. Palygorskite is chemical inert and non polluting. It is used widely in medicine.54 It can inhibit the growth of microorganisms and absorb toxic volatile components. Palygorksite is used as the raw material of cosmetics, and is particularly common in foundation, catapasm and lipstick.55 No information on health risks for this material has been entered into the database.55 It could be included that Y2O3/Pal is environmental friendly when used under appropriate conditions. To the best of our knowledge, the chemical, physical, and toxicological properties of Y2O3/Pal have not been thoroughly investigated. It is very important that investigation on Y2O3 functionalized natural palygorskite as an adsorbent for methyl blue removal in the environmental application. Therefore, Y2O3/Pal was designed, synthesized and characterized. The effects of Y2O3 functionalization on the structure and surface properties of Pal were studied. Also, the mechanism of decolorization was proposed, in which chemical adsorption between Y2O3/Pal and methyl blue was revealed. Results obtained from this work suggested that Y2O3 functionalized Pal might enlarge the application of Pal in the fields of dye wastewater purification.
2. Experimental
2.1 Materials preparation
Y(NO3)3·6H2O (5.11 g) was dissolved in deionized water (200 mL), the ultrasonically dispersed suspension (10.02 g Pal in 100 mL deionized water) was slowly added later. The mixture was stirred at 80 °C for 8 h, dried in an oven at 80 °C overnight. The solid product was put into a crucible and heated at 600 °C for 2 h (at a heating rate of 5 °C min−1) to produce the Y2O3/Pal composite. Pure Y2O3 powders were obtained after calcination of Y(NO3)3·6H2O under the same thermal conditions. Other Pal supported rare earth oxides were synthesized from their nitrates in the same way.2.2 Characterization
X-ray diffraction (XRD) patterns of the samples were recorded by a DX-2700 diffractometer with Cu Kα radiation. Fourier transform infrared (FTIR) spectra were recorded in a Shimadzu FTIR 8120 spectrometer by using the dried KBr disk technique. X-ray photoelectron spectroscopy (XPS) measurements were performed on a spectrometer (K-Alpha 1063, Thermo Fisher Scientific) equipped with an Al Kα X-ray radiation source. The XPS binding energy (BE) was internally referenced to the C1s peak (BE = 284.68 eV). Transmission electron microscopy (TEM) was conducted using a JEM 2100F microscope operated at 200 keV. The zeta-potential was determined by a Zeta Meter System (Backmen-Coulter Delsa 440SX, USA).2.3 Decolorization property
Decolorization of methyl blue (MB) was performed in a glass reactor equipped with a digital thermometer controller. 30 mg sample was added into 300 mL MB solution with magnetic stirring at 400 rpm. The pH value was maintained at 7.1 during the whole process. 5 mL mixture was taken at regular intervals without addition of fresh liquid. The mixture was treated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min before UV-vis measurement. Used samples were collected. The maximum absorbance wavelength (610 nm) was detected and used as concentration index. A 2600 UV/VIS spectrophotometer was used to measure the concentration. The decolorization of MB was calculated by the following equation: Decolorization = 1 − Ce/C0, where C0 and Ce were the initial and final concentrations (mg L−1) of dye, respectively.
000 rpm for 5 min before UV-vis measurement. Used samples were collected. The maximum absorbance wavelength (610 nm) was detected and used as concentration index. A 2600 UV/VIS spectrophotometer was used to measure the concentration. The decolorization of MB was calculated by the following equation: Decolorization = 1 − Ce/C0, where C0 and Ce were the initial and final concentrations (mg L−1) of dye, respectively.
3. Results and discussion
Fig. 1 shows the effect of Y2O3 on the structure of Pal. The characteristic reflection of Pal at 2θ = 8.3° shrunk after calcination at 600 °C. Our previous work indicated that the structure of Pal changed into amorphous SiO2 upon calcination due to the removal of structural water.16,17 Therefore, the XRD spectrum of the Y2O3/Pal showed very low intensity compared with the other spectra.Some other reflections belonging to Pal shrunk or disappeared after modification with Y2O3, which might be caused by the replacement of Si–O by Y–O. Y(NO3)3 was completely decomposed to Y2O3 after calcination at 600 °C for 2 h with a particle size of 18.6 nm calculated from Scherrer's equation, and the Y2O3 content (with a mass ratio of 15% to Pal) was far more than the minimum detection limit of XRD. Therefore, yttrium was supposed to connect with Pal by a Y–O–Si structure or in the form of small particles as reflections belonging to Y2O3 was not obvious.
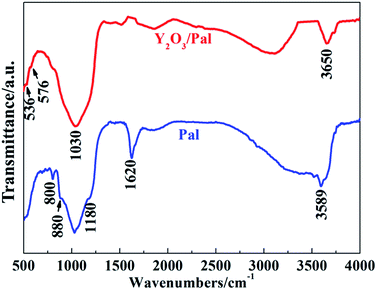
FTIR was employed to characterize the band change of Pal before and after modification. Bands at 800, 880, 1180 and 1030 cm−1 were the different vibrations of Si–O (Fig. 2).17 Bands at 1620, 3589 and 3650 cm−1 were the different OH vibrations of Pal.16,56 Some Si–O vibrations almost disappeared after modification with Y2O3. Band at 536 cm−1 was the Y–O vibration of Y–O–Y, and band at 576 cm−1 was Y–O vibration originated from Y–O–Si. The strength of surface OH groups of Pal reduced in Y2O3/Pal because Y2O3 were bonded on the surface of Pal.
TEM was applied to reveal the distribution of Y2O3 nanoparticles. Y2O3 were uniformly dispersed on Pal with size of 2–5 nm (Fig. 3). The small image inserted in Fig. 3b shows a interplanar spacing of 2.08 Å, coinciding with the (134) plane of Y2O3 (PDF#43-1036). Y2O3 nanoparticles supported by Pal showed regular shape and size compared with the reported results.57,58 These regular particles produced a large number of active sites, which would enhance the dye adsorption performance.
 | ||
| Fig. 3 (a) TEM and (b) HRTEM images of Y2O3/Pal composite (inset is the interplanar spacing), c TEM and d HRTEM images of Pal. | ||
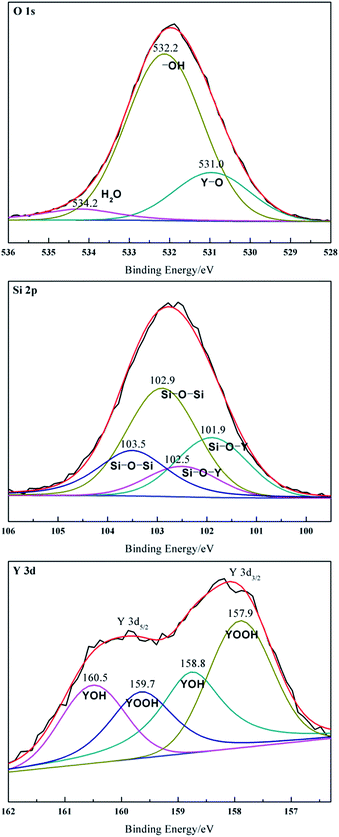
XPS was employed to characterize the surface chemical composition and the oxidation state of the sample. The fitting of O1s region with three peaks indicated three kinds of oxygen species on Y2O3/Pal (Fig. 4). Binding energy at 534.2 eV was assigned to adsorbed water oxygen, 532.2 eV was attributed to hydroxyl groups on the nanoparticle surface, 531.0 eV was caused by lattice oxygen.59 The intensity of the –OH peak was considerably higher than the others. This clearly indicates that the Y2O3/Pal sample has more hydroxyl groups on the surface, which would be beneficial to enhancing the hydrophilicity of Y2O3/Pal.
Binding energy of Y2O3 (Y3d5/2) was reported at 156.8,29 156.4 (ref. 59) or 156.2 eV.60 However, none of them was observed in this work. The peaks of the four yttrium contributions showed higher chemical shift. Water molecules would be strongly adsorbed on Y2O3 and react with bulk oxide layers to form a strongly hydrated species (in the form of YOOH or Y(OH)3) even at room temperature. The Y3d5/2 binding energy of vanadium–yttrium hydrates (158.6 eV) was higher than that of Y2O3 (156.8 eV) because of the presence of hydrogen.61 Therefore, the binding energies at 158.8 (3d5/2) and 160.5 (3d3/2) eV with a spin–orbit splitting of 1.7 eV were assigned to the yttrium-hydroxyl.
The binding energies at 157.9 (3d5/2) and 159.7 (3d3/2) eV with a spin–orbit splitting of 1.8 eV indicated that the chemical bond of YOOH groups on the nanoparticle surface was more than that of YOH. In addition, binding energies at 102.9 and 103.5 eV belonged to Si–O–Si. Si was linked to surface Y by a Si–O–Y chemical bond, suggested by the lower binding energies at 101.9 and 102.5 eV.62,63
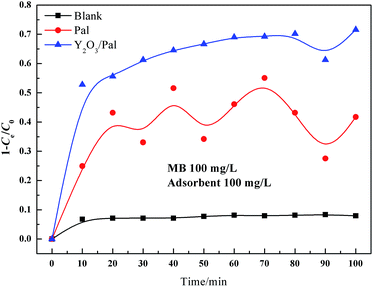
The decolorization of MB (Fig. 5) was used to evaluate the adsorption performance of samples. Y2O3/Pal showed better performance than that of Pal. The T50 (time that used to reach the decolorization of 50%) of Y2O3/Pal was in 10 min while that of Pal was about 40 min. In addition, the decolorization of Y2O3/Pal was much more stable than that of Pal. The decolorization performance and its rate were both enhanced by Y2O3 modification.
Our previous work has indicated that the zeta potential of Pal decreased from +3.2 to −14.8 mV with increasing pH. Y2O3 modification greatly increased the number of negatively charged groups as Y2O3/Pal showed lower negative zeta potential than that of Pal (Fig. 6). Therefore, the good decolorization performance was impossible to benefit from the electrostatic adsorption between Y2O3/Pal and MB molecule as Y2O3/Pal was negatively charged at pH 7.1 and MB molecules also negatively charged in one of the –SO3 groups.
The performance of sample at different temperatures was showed in Fig. 7. Increasing temperature benefited the adsorption, which means the adsorption process was endothermic. The dye adsorption curves of sample at 60 °C were simulated with Langmuir, Freundlich and Dubinin–Radushkevich equations64 (Fig. 8 and Table 1). The curve was best simulated with Langmuir equation, suggesting the monolayer chemical adsorption of MB. The maximum adsorption capacity is up to 1579.06 mg g−1, exhibiting potential applications in wastewater treatment.
| Adsorbent | T/°C | Langmuir adsorption isotherm qe = qmKLCe/(1 + KLCe) | Freundlich adsorption isotherm qe = KFCe1/n | Dubinin–Radushkevich adsorption isotherm ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qmax − βε2; ε = RT(1 + 1/Ce); E = 1/(2β)1/2 qmax − βε2; ε = RT(1 + 1/Ce); E = 1/(2β)1/2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL (L mg−1) | qm (mg g−1) | R2 | n | Kf (mg g−1) | R2 | β (mol2 kJ−1) | E (kJ mol−1) | R2 | ||
| a Note: qe: adsorbing capacity (mg g−1), qm: saturated extent of adsorption (mg g−1), KL: Langmuir adsorption equilibrium constant (L mg−1); KF: Freundlich adsorption coefficient, n: Freundlich constant; β: adsorption coefficient, ε: the adsorption potential energy, E: characteristic energy. | ||||||||||
| Y2O3/Pal | 60 | 0.0077 | 1579.06 | 0.9879 | 1.054 | 12.18 | 0.9840 | 0.5788 | 0.9295 | 0.8183 |
As the Y2O3 modification can increase the number of negatively charged groups of Pal, Y2O3/Pal can be used in removing other toxicants from the environment. The capacity of adsorbing heavy metal cations of Y2O3/Pal is obviously higher than that of Pal. The increase in the surface negatively charged groups could greatly enhance the removal efficiency of heavy metal cations. Also, Y2O3 modification have effect on capturing cationic surface-active agent from water.
Molecular sieve and activated carbon are kind of common adsorbent that used in wastewater treatment besides clay minerals. Y2O3/Pal showed faster and more stable performance than that of Pal in this work. The treatment of molecular sieve and activated carbon in wastewater is mainly a process of physical adsorption, the rate of which is usually slower than that of chemical adsorption. Also, the desorption can be hardly avoid because of dynamic equilibrium of physical adsorption.
Y2O3/Pal showed nearly 90% decolorization in the first run of recycle test (Fig. 9). The 30 min decolorization in all recycling runs was higher than 80%. Therefore, Y2O3/Pal was stable and could be reused in the decolorization of MB. Additionally, increasing Y2O3 content benefited the performance of Y2O3/Pal (Fig. 10a) and caused more replacement of Si–O structure by Y–O structure (Fig. 10b). Meanwhile, the adsorption performance of Y2O3/Pal was enhanced when the pH was increased (Fig. 10c). Palygorskite modified by other rare earth oxides such as CeO2 and Eu2O3 with ratio of rare earth oxides to Pal of 15 wt% also enhanced the performance of Pal (Fig. 10d). These results demonstrate that palygorskite attaches rare earth oxides such as Y2O3, CeO2 and Eu2O3 could provide a kind of novel adsorbents for enhanced dye adsorption. However, as all we know, rare earth compounds may cause delayed blood clotting leading to hemorrhages. Inhalation of rare earths may cause sensitivity to heat, itching, and increased awareness of odor and taste, coagulation abnormalities, gastrointestinal disturbance. Inhalation may result in asthma, cough, muscles, damage to the lungs.53 In addition, although Y2O3 is not radioactive, palygorskite has the possibility as radioactive elements might integrated into its structure because of geologic action. Therefore, we had to think carefully about whether this material is suitable for environmental pollutant removal. Considering that clay minerals are plastic substances, Pal can be made of honeycomb or other shapes after sintering,17,18 therefore, Y2O3/Pal sintered is stable and can be easily collected from wastewater and recycled. In addition, although Y2O3 is stable in neutral water or alkali solution, it may be soluble in acidic solution to cause environmental pollution in some extent. Therefore, the solubility of Y2O3/Pal in acidic solution need to be measured, and radiation detection should be employed to ensure the safety of Pal in the future work.
 | ||
| Fig. 10 Effects of (a) Y2O3 mass ratio, (c) pH value and (d) rare earth oxides at Y2O3, CeO2, Eu2O3 to Pal of 15 wt% on the decolorization of MB, (b) XRD patterns of Y2O3/Pal at different mass ratio. | ||
4. Conclusions
A novel adsorbent was designed and synthesized, which showed stable and rapid decolorization performance for methyl blue. Y2O3 nanoparticles played an important role in enhancing the performance because they increased the number of surface bonds of Pal and could form chemical bond with dye molecule. Therefore, methyl blue was decolorized in a stable and rapid way by chemical adsorption. Besides Y2O3/Pal, Pal supported CeO2 and Eu2O3 also have good performance for enhanced dye adsorption. Furthermore, the Y2O3/Pal composite could be easily separated by centrifugation, and could be reused in the purification of dye wastewater.Acknowledgements
This work was supported by the National Science Fund for Distinguished Young Scholars (51225403), the National Natural Science Foundation of China (51374250), the Specialized Research Fund for the Doctoral Program of Higher Education (20120162110079) and Hunan Provincial Innovation Foundation for Postgraduates (CX2012B122).References
- J. M. Aquino, R. C. Rocha-Filho, P. C. C. Sáez and M. A. Rodrigo, Environ. Sci. Pollut. Res., 2014, 21, 8442–8448 CrossRef CAS PubMed.
- H. Olvera-Vargas, N. Oturan, C. T. Aravindakumar, M. M. S. Paul, V. K. Sharma and M. A. Oturan, Environ. Sci. Pollut. Res., 2014, 21, 8379–8386 CrossRef CAS PubMed.
- Z. Wang, C. Fang and M. Megharaj, ACS Sustainable Chem. Eng., 2014, 2, 1022–1025 CrossRef CAS.
- M. Hakimelahi, M. R. A. Moghaddam and S. H. Hashemi, Biotechnol. Bioprocess Eng., 2012, 17, 875–880 CrossRef CAS.
- H.-J. Wang, C.-F. Wang, Y.-Y. Sun and Y. Cao, Chem. Eng. J., 2012, 209, 442–450 CrossRef CAS.
- R. M. Jain, K. H. Mody, J. Keshri and B. Jha, Mar. Pollut. Bull., 2014, 84, 83–89 CrossRef CAS PubMed.
- L. Liu, J. Ding, C. Huang, M. Li, H. Hou and Y. Fan, Cryst. Growth Des., 2014, 14, 3035–3043 CAS.
- A. A. P. Mansur, H. S. Mansur, F. P. Ramanery, L. C. Oliveira and P. P. Souza, Appl. Catal., B, 2014, 158, 269–279 CrossRef.
- C. Tan, G. Zhu, M. Hojamberdiev, K. Okada, J. Liang, X. Luo, P. Liu and Y. Liu, Appl. Catal., B, 2014, 152, 425–436 CrossRef.
- M. Tokumura, T. Katoh, H. Ohata and Y. Kawase, Ind. Eng. Chem. Res., 2009, 48, 7965–7975 CrossRef CAS.
- M. A. Al-Ghouti, Y. S. J. Li, N. Al-Laqtah, G. Walker and M. N. M. Ahmad, J. Hazard. Mater., 2010, 176, 510–520 CrossRef CAS PubMed.
- M. Greluk and Z. Hubicki, Chem. Eng. J., 2013, 215–216, 731–739 CrossRef CAS.
- Z. Jiang, J. Xie, D. Jiang, Z. Yan, J. Jing and D. Liu, Appl. Surf. Sci., 2014, 292, 301–310 CrossRef CAS.
- E. Kharlampieva and S. A. Sukhishvili, Langmuir, 2004, 20, 9677–9685 CrossRef CAS PubMed.
- N. Rajendiran and R. K. Sankaranarayanan, Carbohydr. Polym., 2014, 106, 422–431 CrossRef CAS PubMed.
- C. Huo and H. Yang, Appl. Clay Sci., 2010, 50, 362–366 CrossRef CAS.
- X. He, A. Tang, H. Yang and J. Ouyang, Appl. Clay Sci., 2011, 53, 80–84 CrossRef CAS.
- C. Huo and H. Yang, J. Colloid Interface Sci., 2012, 384, 55–60 CrossRef CAS PubMed.
- D. N. Taha and I. S. Samaka, J. Oleo Sci., 2012, 61, 729–736 CrossRef CAS.
- Y. G. Peng, D. J. Chen, J. L. Ji, Y. Kong, H. X. Wan and C. Yao, Environ. Prog. Sustainable Energy, 2013, 32, 1090–1095 CrossRef CAS.
- W. T. Wu, Z. F. Nie, F. I. Tan, F. Xu, L. Xu and K. I. Gao, Polym. Polym. Compos., 2013, 21, 565–572 CAS.
- X. G. Wang, S. Y. Lu, C. M. Gao, X. B. Xu, X. J. Zhang, X. Bai, M. Z. Liu and L. Wu, Chem. Eng. J., 2014, 252, 404–414 CrossRef CAS.
- Y. Guan, H. Qian, J. Q. Guo, S. G. Yang, X. Wang, S. M. Wang and Y. S. Fu, Appl. Clay Sci., 2015, 114, 124–132 CrossRef CAS.
- H. Zhao, F. X. Qiu, J. Yan, J. Wang, X. Li and D. Y. Yang, Appl. Clay Sci., 2016, 121, 137–145 CrossRef.
- E. Alvarez-Ayuso and A. Gardia-Sanchez, J. Hazard. Mater., 2007, 147, 594–600 CrossRef CAS PubMed.
- Y. Kong, J. X. Wei, Z. L. Wang, T. Sun, C. Yao and Z. D. Chen, J. Appl. Polym. Sci., 2011, 122, 2054–2059 CrossRef CAS.
- C. Yao, Y. Y. Xu, Y. Kong, W. J. Liu, W. J. Wang, Z. L. Wang, Y. Wang and J. L. Ji, Appl. Clay Sci., 2012, 67–68, 32–35 CrossRef CAS.
- G. Jia, H. You, Y. Song, Y. Huang, M. Yang and H. Zhang, Inorg. Chem., 2010, 49, 7721–7725 CrossRef CAS PubMed.
- W. O. Gordon, B. M. Tissue and J. R. Morris, J. Phys. Chem. C, 2007, 111, 3233–3240 CAS.
- J. Yang, Z. Quan, D. Kong, X. Liu and J. Lin, Cryst. Growth Des., 2007, 7, 730–735 CAS.
- T. Ishihara and K. Goto, Catal. Today, 2011, 164, 484–488 CrossRef CAS.
- Y. Kuroda, H. Hamano, T. Mori, Y. Yoshikawa and M. Nagao, Langmuir, 2000, 16, 6937–6947 CrossRef CAS.
- X. He and H. M. Yang, Dalton Trans., 2015, 44, 1673–1679 RSC.
- X. He, Q. Yang, L. J. Fu and H. M. Yang, Funct. Mater. Lett., 2015, 8, 1550056 CrossRef CAS.
- J. Jin, L. J. Fu, J. Ouyang and H. M. Yang, Sci. Rep., 2015, 5, 12429 CrossRef PubMed.
- X. Y. Li, J. Ouyang, Y. H. Zhou and H. M. Yang, Sci. Rep., 2015, 5, 13736 CrossRef PubMed.
- S. Y. Liu and H. M. Yang, Energy Technol., 2015, 3, 77–83 CrossRef CAS.
- H. L. Lun, J. Ouyang, A. D. Tang and H. M. Yang, Nano, 2015, 10, 1550078 CrossRef CAS.
- K. Peng, J. Y. Zhang, H. M. Yang and J. Ouyang, RSC Adv., 2015, 5, 66134–66140 RSC.
- X. Li and A. D. Tang, RSC Adv., 2016, 15585–15591 RSC.
- K. Peng, L. J. Fu, J. Ouyang and H. M. Yang, Adv. Funct. Mater., 2016 DOI:10.1002/adfm.201504942.
- K. Peng, L. J. Fu, H. M. Yang and J. Ouyang, Sci. Rep., 2016, 6, 19723 CrossRef CAS PubMed.
- Y. Zhang, A. D. Tang, H. M. Yang and J. Ouyang, Appl. Clay Sci., 2016, 119, 8–17 CrossRef CAS.
- Z. Zhou, J. Ouyang, H. M. Yang and A. D. Tang, Appl. Clay Sci., 2016, 121, 63–70 CrossRef.
- Q. Yang, M. Long, L. Tan, Y. Zhang, J. Ouyang, P. Liu and A. D. Tang, ACS Appl. Mater. Interfaces, 2015, 7, 12719–12730 CAS.
- M. Long, L. Tan, H. T. Liu, Z. He and A. D. Tang, Biosens. Bioelectron., 2014, 59, 243–250 CrossRef CAS PubMed.
- W. J. Ding, H. M. Yang and J. Ouyang, RSC Adv., 2015, 5, 67184–67194 RSC.
- L. J. Fu, C. L. Huo, X. He and H. M. Yang, RSC Adv., 2015, 5, 20414–20423 RSC.
- C. C. Li, L. J. Fu, J. Ouyang, A. D. Tang and H. M. Yang, Appl. Clay Sci., 2015, 115, 212–220 CrossRef CAS.
- K. Peng, H. M. Yang and J. Ouyang, Powder Technol., 2015, 286, 678–683 CrossRef CAS.
- Y. Y. Xie, A. D. Tang and H. M. Yang, Nano, 2015, 10, 155005 Search PubMed.
- W. J. Ding, J. Ouyang and H. M. Yang, Powder Technol., 2016, 292, 169–175 CrossRef CAS.
- http://www.guidechem.com/msds/1314-36-9.html.
- https://en.wikipedia.org/wiki/Palygorskite.
- http://www.mindat.org/min-3072.html#.
- A. L. Valant, N. Bion, F. Can, D. Duprez and F. Epron, Appl. Catal., B, 2010, 97, 72–81 CrossRef.
- B. Guo, M. Mukundan and H. Yim, Powder Technol., 2009, 191, 231–234 CrossRef CAS.
- S. A. Song, K. Y. Jung and S. B. Park, Langmuir, 2009, 25, 3402–3406 CrossRef CAS PubMed.
- X. C. Zhang, H. M. Yang and A. D. Tang, J. Phys. Chem. B, 2008, 112, 16271–16279 CrossRef CAS PubMed.
- G. B. Sun, K. Hidajat, X. S. Wu and S. Kawi, Appl. Catal., B, 2008, 81, 303–312 CrossRef CAS.
- V. Bondarenka, S. Grebinskij, S. Kaciulis, G. Mattogno, S. Mickevicius, H. Tvardauskas, V. Volkov and G. Zakharova, J. Electron Spectrosc. Relat. Phenom., 2001, 120, 131–135 CrossRef CAS.
- G. Liu, G. Hong and D. Sun, J. Colloid Interface Sci., 2004, 278, 133–138 CrossRef CAS PubMed.
- C. M. Hessel, A. T. Heitsch and B. A. Korgel, Nano Lett., 2010, 10, 176–180 CrossRef CAS PubMed.
- Y. Shao, Q. Sun, Q. Zhang, L. Li and P. Liu, Acta Sci. Circumstantiae, 2014, 34, 3011–3016 CAS.
Footnote |
| † These authors contributed equally to the research. |
| This journal is © The Royal Society of Chemistry 2016 |