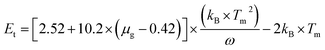Enhanced luminescence and abnormal thermal quenching behaviour investigation of BaHfSi3O9:Eu2+ blue phosphor co-doped with La3+–Sc3+ ion pairs
Shuangyu Xin and
Ge Zhu*
College of New Energy, Bohai University, Jinzhou, Liaoning 121000, P. R. China. E-mail: zhuge@bhu.edu.cn; Fax: +86-416-3400708; Tel: +86-416-3400708
First published on 20th April 2016
Abstract
An emission and thermal stability enhanced blue emitting phosphor BaHfSi3O9:Eu2+ co-doped with La3+–Sc3+ ion pairs was successfully synthesized. The phase purity and characteristic luminescent properties were investigated in detail. The results indicate that BaHfSi3O9:Eu2+ co-doped with La3+–Sc3+ ion pairs phosphor has strong excitation around 405 nm and can exhibit blue emission centered at 476 nm. The emission intensity of BaHfSi3O9:Eu2+ can be gradually enhanced via introducing La3+ and Sc3+ ions and the mechanism is studied. Meanwhile, the improved thermal stability can be realized. A possible principle is proposed by the simplified energy level diagram and is further proved through the thermoluminescence curve analysis and the time-waited temperature dependent emission spectra measurement.
1. Introduction
During the past few decades, solid state lighting based on light emitting diodes (LEDs) technology has gradually become an important technology for generating white light emission and has made significant contribution to human life on quality and productivity.1,2 Nowadays, the luminescent efficiency achieved by LEDs has exceeded 260 lm W−1, which is far superior to those of traditional light sources, such as Edison-style incandescent lamps (∼16 lm W−1) and fluorescent lamps (<100 lm W−1).3 In addition, LEDs have numerous advantages over the traditional light sources, including energy-saving, small size, fast switching, high lifetime and being environmentally friendly, which contribute LEDs to be applied in not only point light sources but also illumination equipment and back-lighting of display devices.4 However, in order to become an ideal role in solid state lighting, several technical challenges still need to be solved. The major problem in the current white LEDs generated by the blue LED chip combining with a yellow phosphor (Y,Gd)3(Al,Ga)5O12:Ce3+ (YAG:Ce3+) is the low color-rendering index (CRI) and high correlated color temperature (CCT) due to the lack of red component.5,6 To solve this problem, more attention has been paid on the white light generation based on the ultraviolet/near-ultraviolet (UV/n-UV) LED chips coated with red, green, and blue emitting (RGB) phosphors.7,8 Undoubtedly, phosphors play a crucial role in producing high quality white light. Nevertheless, the degradation of luminous efficiency due to the strong reabsorption of the blue emission by the green or red-emitting phosphors remains to be one of the problems within this system.9 Hence, there is an urgent need to develop new n-UV excitable blue emission phosphors with high efficiency or improve the current potential phosphors. Moreover, with the increasing market need for high-power operation, the employed phosphors should have strong thermal stability and high initial intensity to resist the high temperature in the work process.10 Thus, it is desirable to realize excellent thermal stability and high emission intensity in phosphors and find out the potential mechanism.As an important candidate for luminescent host materials, silicates are of great interest for their high physical, chemical stability and various crystal structures. The blue-emitting silicate phosphor BaHfSi3O9:Eu2+ was first synthesized by Wang et al. in 2011, which can emit blue emission at 475 nm with high quantum efficiency (∼50%) and good thermal stability (keep 86% at 150 °C).11 More importantly, the maximal excitation of BaHfSi3O9:Eu2+ was found to be 405 nm, which was infrequent in oxide based blue phosphors and was well matched with the current n-UV (405 nm) LED chip. However, to apply in high-power LEDs, higher initial emission intensity and better thermal stability are anticipated. In this paper, La3+ and Sc3+ ion pairs were co-doped to form Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors. The enhanced luminescence was realized and investigated. Moreover, the abnormal thermal quenching property was found and the potential mechanism was proposed and proved through thermoluminescence and the temperature dependent emission spectral analysis.
2. Experimental
2.1 Materials and synthesis
All the samples were synthesized by high temperature solid method. BaCO3 (A.R), La2O3 (A.R), Sc2O3 (A.R), HfO2 (99.99%), SiO2 (A.R), and Eu2O3 (99.99%) were mixed stoichiometrically and ground thoroughly in an agate mortar. The mixture was put into an alumina crucible and sintered for 2 h at 800 °C in air. Then the preheated mixture was ground again and fired to 1400 °C for 6 h in an alumina crucible under reducing atmosphere of N2–H2 (8%) in horizontal tube furnaces.2.2 Measurements and characterization
The crystal structure was identified by using a Rigaku D/Max-2400 X-ray diffractometer (XRD) with Ni-filtered Cu Kα radiation. The photoluminescence excitation and emission spectra of the samples at room temperatures were measured by using an FL-1039 (Horiba Jobin Yvon) fluorescence spectrophotometer equipped with a 450 W xenon light source. All of the measurements were performed at room temperature. High-temperature luminescence intensity measurements were carried out by using an aluminum plaque with cartridge heaters; the temperature was measured by thermocouples inside the plaque and controlled by a standard TAP-02 high-temperature fluorescence controller. The thermoluminescence curves were measured by a FJ-427A1 meter (Beijing Nuclear Instrument Factory) with a heating rate of 1 K s−1. Before the measurement, the samples were irradiated with ultraviolet light (365 nm) for 10 min.3. Results and discussion
3.1 Phase purity analysis
Fig. 1 shows the Rietveld structural refinement of powder diffraction patterns of Ba0.97La0.01Hf0.99Sc0.01Si3O9:0.02Eu2+ phosphors.12 The red solid lines and black crosses are calculated patterns and experimental patterns, respectively. The pink short vertical lines show the position of Bragg reflections of the calculated pattern. The difference between the experimental and calculated patterns is plotted by green line at the bottom. The structure parameters reported on BaHfSi3O9 are used as initial parameters in the Rietveld analysis and the residual factors are Rwp = 19.65%, and Rp = 15.32%.11 It can be seen that all the obtained experimental XRD patterns are well fitted with the calculated XRD patterns except some tiny impurity peaks around 28.3 and 32.8 degree, which is identified to be HfO2 according to JCPDF card (no. 65-1142). The observed tiny impurity phase is in expectation since it has been reported that the single phased BaHfSi3O9 is hard to be synthesized.11 In addition, a control experiment of Eu ion doped HfO2 has also been taken by the same synthesized condition in order to eliminate the impurity effect and the result shows that Eu ion cannot be introduced into the host lattice and be reduced to Eu2+ ions due to the large difference in radius and valency. No emission can be detected from 400 to 600 nm under 405 nm excitation (not shown here), indicating that the impurity phase HfO2 will not affect the luminescent investigation. A series of XRD patterns of Ba1−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) were measured. The new impurity phase could be detected when x is above to 0.02. As a result, the following discussion on the luminescent property is based on the phosphors with doping concentration below 0.02. | ||
| Fig. 1 Power XRD patterns for Rietveld structure analysis of the selected Ba0.97La0.01Hf0.99Sc0.01Si3O9:0.02Eu2+ sample. | ||
3.2 Enhanced photoluminescence investigation
Fig. 2 illustrates the normalized excitation spectra of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors monitored at 476 nm. It can be seen that the excitation spectra show a broad excitation band ranging from 250 to 450 nm, especially has strong intensity around 405 nm, which indicates that Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphor can serve as a promising candidate for n-UV pumped white LEDs. The unresolved broad band is assigned to the transition between the ground-state 4f7 and the crystal-field split 4f65d configuration of Eu2+ ion.13 The inset shows the photograph of the synthesized series samples irradiated by sunlight and 365 UV lamp. The peak green body color of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) samples can be attributed to their strong absorption in UV to blue region. Meanwhile, bright blue emission could be realized in the obtained samples when irradiated under a 365 UV lamp.Fig. 3 shows the emission spectra of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors excited at 405 nm. Under 405 nm excitation, the Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ phosphors exhibit an intense blue emission and the corresponding emission spectrum consists of a broad emission band ranging from 420 nm to 600 nm due to the electric-dipole-allowed transition from the 5d excited state to the 4f ground state of the Eu2+ ion.13 As increasing the doping contents x, it is interesting that the emission intensity of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ phosphors is found to be increased until x = 0.01. The enhanced luminescent emission intensity can be attributed to the compression effects when La3+ and Sc3+ are co-doped into the host lattice (BaO6 and HfO6) since the effective ion radius of La3+ (six coordination, 1.032 Å) is smaller than that of Ba2+ (six coordination, 1.35 Å) while Hf4+ (six coordination, 0.71 Å) and Sc3+(six coordination, 0.745 Å) have similar ion radius.14,15 A significant strain may arise in the lattice around the Eu2+ ions, and may limit the stability of the Eu2+ ions which has been prior incorporated into the lattice.16 In other words, the number of effective luminescent centers is increased through the limitation effect. However, when x is increased to 0.01, the luminescent centers have already reached to maximum. The more doping contents will cause the quenching of Eu2+ ions. Moreover, with the increase of doping contents x, the emission spectrum is found to be moved from 476 nm to 481 nm. The red-shift behaviour can also be well explained in terms of an increase in the crystal field which is caused by the compression effects.17,18
 | ||
| Fig. 3 The emission spectra of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors excited at 405 nm. | ||
3.3 The abnormal thermal quenching behaviour investigation
It is required that the thermal quenching of phosphors should be small and must sustain emission efficiency at high temperatures over a long term while they are used in W-LEDs, typically in high-power ones, where the operation temperature could reach up to 450 K. Till now, few oxide-based phosphors have been reported that their luminescence emission intensity can kept up to 90% at 450 K. Fig. 4 illustrates the temperature-dependent luminescent properties of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ phosphor measured between 293 and 503 K under 405 nm excitation. The emission intensity of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) as a function of the sample temperature is also plotted in the inset of Fig. 4. For comparison, the thermal quenching behaviour of the commercial blue phosphor BaMgAl10O17:Eu2+ (BAM:Eu2+) has also been measured. Generally, the increased temperature can amplify the population of higher vibration levels, the density of phonons and the probability of non-radiative transfer (energy migration to defects), as a result the emission intensity would gradually decrease. In this case, it is worthwhile to note that when x = 0, the thermal quenching behaviour is normal and a little worse than that of the commercial BAM:Eu2+. When La3+ and Sc3+ ions are introduced, the emission intensity of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ is found to decrease gradually before 413 K and rapidly increase when the temperature is above 413 K, as shown in Fig. 4. In addition, accompanied by the abnormal thermal quenching behaviour, it is interesting that the emission wavelength shows slight blue shifting with raising temperature, which can be explained by thermally active phonon-assisted excitation from lower energy sublevel to higher-energy sublevel in the excited states of Eu2+.19,20 The surprised increased emission intensity and the thermal quenching behaviour are quite abnormal and to the best of our knowledge, there is no report about the reasonable explanation on similar phenomenon. To verify this unusual behaviour, Eu2+-doped Ba0.98−xLaxHf1−xScxSi3O9 phosphors with other contents (x = 0, x = 0.01 and x = 0.02) are also measured, and they show similar results, as shown in the inset. It should be noted that with raising the doping contents, the increasing tendency of the abnormal emission intensity became apparent. Thus, it can be conjectured that the abnormal thermal behaviour should be related to the cation substitution in the current host lattice.3.4 The mechanism investigation of the abnormal thermal quenching behaviour
The formation of defect is of considerable importance for the thermodynamic stability and energy level distributions in materials.21,22 When La3+ and Sc3+ ions were introduced to replace Ba2+ and Hf4+ ions, it is ostensibly charge balanced and actually it would unavoidably create defects during the solid state reaction and some traps may also be generated in this process. In theory, the more impurity ions are introduced, the more traps will be generated. The energy exchange processes from traps to traps or traps to emission centers can affect luminescence property of phosphors, such as the long lasting phosphorescence (LLP) phosphors.23,24 Generally, the information about the traps and the trapping levels can be obtained from the thermoluminescence (TL) curve. The significant role of various traps in the obtained samples has been recognized through TL measurement, as illustrated in Fig. 5. The TL curve of the un-doped BaHfSi3O9:0.02Eu2+ sample shows some weak bands at about 455 K, 520 K and 570 K, which may due to the generated oxygen vacancy during the sintering process at high temperature. When La3+ and Sc3+ ions are introduced, it is obvious that a strong trap band is observed around 425 K, associated with two weak trap bands around 498 and 554 K. Moreover, the intensity of trap bands is found to be sharply increased as rising the doping content x, indicating that the three traps are created by introducing La3+ and Sc3+ ions into BaHfSi3O9:0.02Eu2+ phosphors. The effective TL band is determined to be the one located at 425 K, since the other two trap bands are too weak and out of the thermal quenching measuring range, which are too deep to free the trapped charge carries and would not have an effect on the thermal behavior.25 As illustrated in the inset, the TL curve of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ can be well fitted into three peaks, named T1, T2 and T3, and only trap T1 will have contribution to the abnormal thermal behaviour. In order to further estimate the trap state, the trap depths (Et) and trap densities (n0) are calculated by using Chen's equation,26| n0 = ω × Im/{β × [2.52 + 10.2 × (μg − 0.42)]} |
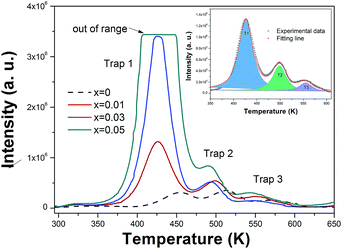 | ||
| Fig. 5 The TL curves of Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors; the inset shows the peak fitting result of TL curve of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ phosphor. | ||
| Trap | Tm (K) | Glow-peak parameters | TL parameters | ||||
|---|---|---|---|---|---|---|---|
| τ | δ | ω | μg | Et (eV) | n0 | ||
| 1 | 425 | 21 | 21 | 42 | 0.5 | 0.186 | 1.63 × 107 |
| 2 | 498 | 18 | 18 | 36 | 0.5 | 0.303 | 5.36 × 106 |
| 3 | 554 | 20 | 20 | 40 | 0.5 | 0.337 | 2.24 × 106 |
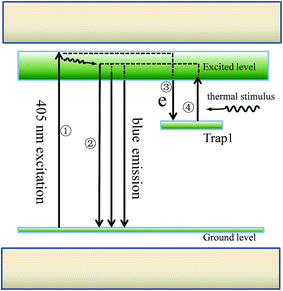
Fig. 6 illustrates the possible principle diagram of the observed abnormal thermal behaviour. At room temperature, while exciting the sample by 405 nm light, the electrons can be excited to higher excited state level of Eu2+ through path ① and relaxed to the lower excited state level then return to ground state level through path ②, resulting in a blue emission at 476 nm. This is the common process at room temperature. At high temperature, since there exists some traps in the current samples, when the electrons are excited to excited state level, some of the “active” electrons can be captured by the traps (Tape 1, for example) through path ③ and do not return to the ground state level. When the samples are heated to an appropriate temperature (413 K, for example), with the effect of the thermal stimulation, the “stored” electrons in the traps can be stimulated to the excited level through path ④ and finally return to ground state level. The higher the temperature is, the more electrons will be stimulated to excited level through path ④ to path ②. At the same time, it should be noted that the higher temperature will cause more energy loss of the electrons during the relaxation process attributed to the enhanced non-radiative transition, resulting in the reduced electrons in path ② from path ①. Thus, the thermal quenching behaviour should be the coupling contribution by the above two effection. In the current system, the electrons population stimulated from path ④ to path ② is higher than that lost in the relaxation process from path ① to path ②, leading to the observed increased emission intensity and the abnormal thermal quenching behaviour.
Generally, the temperature dependent luminescent property of phosphors is measured when the sample is heated to a certain temperature and kept at this temperature for at least one minute to insure the uniform heat of the sample. In this case, it is worthwhile to note that attributed to the deep traps in the sample, it will take some time for the electrons captured by the traps to be stimulated to excited level at high temperatures. The principle is similar to that in LLP phosphors. Thus in order to further prove the above mechanism of the abnormal thermal quenching behaviour, the thermal stability property of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ phosphor dependent on different waiting time is investigated, as shown in Fig. 7. It can be seen that the emission intensity is found to decrease gradually with increasing the waiting time. This phenomenon is reasonable and expected because the electrons will be stimulated from traps and return to the ground state during the waiting time and the longer the waiting time, the more electrons will be released. As a result, the emission intensity measured after the waiting time is found to decrease gradually. When the heat time is above 10 min, the emission intensity almost keeps unchanged, indicating that almost all the electrons are released at this moment and the traps do not affect the thermal quenching behaviour any more. The above analysis further proves the proposed mechanism for the abnormal thermal quenching behaviour. Anyway, the enhanced luminescent emission intensity and thermal stability is beneficial for phosphors for applied in high-power white LEDs.
 | ||
| Fig. 7 The temperature dependent emission spectra of Ba0.975La0.005Hf0.995Sc0.005Si3O9:0.02Eu2+ measured at 413 K after waiting different time. | ||
4. Conclusion
In summary, a blue emitting phosphor BaHfSi3O9:0.02Eu2+ co-doped with La3+ and Sc3+ ions was successfully synthesized. The phase purity and the characteristic luminescent properties were investigated in detail. The excitation spectra showed that Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors had strong excitation ranging from 280 to 450 nm, with dominated excitation around 405 nm. Upon 405 nm excitation, the emission spectra showed broad emission band centered at 476 nm, corresponding to the electric-dipole-allowed transition from the 5d excited state to the 4f ground state of the Eu2+. The increased emission intensity was observed via the increase of the doping content, due to the variation of lattice environment around Eu2+. Moreover, the thermal stability was significantly increased via the introduction of La3+ and Sc3+ ions when the temperature was over 140 °C. The abnormal thermal quenching behaviour of Ba0.98−xLa-Hf1−xScx–Si3–O9–:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors was investigated and the mechanism was discussed in detail, which was determined to be attributed to the formed defects when La3+ and Sc3+ ions were introduced into the host lattice. A possible principle was proposed and proved through the analysis of TL curve and the time-waited temperature dependent emission spectra at 413 K. The results showed that the emission and thermal stability enhanced Ba0.98−xLaxHf1−xScxSi3O9:0.02Eu2+ (0 ≤ x ≤ 0.02) phosphors could serve as a good candidate for high-power white LEDs.References
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274 CrossRef CAS PubMed.
- S. Pimputkar, J. S. Speck, S. P. DenBaars and S. Nakamura, Nat. Photonics, 2009, 3, 180 CrossRef CAS.
- J. Ryou and R. D. Dupuis, Opt. Express, 2011, 19, A897 CrossRef PubMed.
- H. Yamamoto, White LED Phosphors: the Next Step. Proc. SPIE 7598, Optical Components and Materials, 2010, VII, 759808 Search PubMed.
- M. Roushan, X. Zhang and J. Li, Angew. Chem., Int. Ed., 2012, 124, 451 CrossRef.
- A. A. Setlur, W. J. Heward, Y. Gao, A. M. Srivastava, R. G. Chandran and M. V. Shankar, Chem. Mater., 2006, 18, 3314 CrossRef CAS.
- C. C. Lin, Z. R. Xiao, G. Y. Guo, T. S. Chan and R. S. Liu, J. Am. Chem. Soc., 2010, 132, 3020 CrossRef CAS PubMed.
- S. E. Brinkley, N. Pfaff, K. A. Denault, Z. Zhang, H. T. Hintzen, R. Seshadri, S. Nakamura and S. P. DenBaars, Appl. Phys. Lett., 2011, 99, 241106 CrossRef.
- K. Sakuma, N. Hirosaki and R. J. Xie, J. Lumin., 2007, 126, 843 CrossRef CAS.
- M. H. Crawford, IEEE J. Sel. Top. Quantum Electron., 2009, 15, 1028 CrossRef CAS.
- D. Wang, Y. Wu and T. Chen, J. Mater. Chem., 2011, 21, 18261 RSC.
- A. C. Larson and R. B. Von Dreele, General Structure Analysis System (GSAS) Los Alamos Nat. Lab, Report, LAUR, 2000, pp. 86–748 Search PubMed.
- G. Lee, J. Y. Han, W. B. Im, S. H. Cheong and D. Y. Jeon, Inorg. Chem., 2012, 51, 10688 CrossRef CAS PubMed.
- W. Huang, F. Yoshimura, K. Ueda, Y. Shimomura, H. Sheu, T. Chan, C. Chiang, W. Zhou and R. Liu, Chem. Mater., 2014, 26, 2075 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, 32, 751 CrossRef.
- Y. Gu, Q. Zhang, Y. Li, H. Wang and R. Xie, Mater. Lett., 2009, 63, 1448 CrossRef CAS.
- Y. Q. Li, A. C. A. Delsing, G. de With and H. T. Hintzen, Chem. Mater., 2005, 17(50), 3242 CrossRef CAS.
- C. Wang, Z. Zhao, Q. Wu, G. Zhu and Y. Wang, Dalton Trans., 2015, 44, 10321 RSC.
- Z. Xia, R. Liu, K. W. Huang and V. Drozd, J. Mater. Chem., 2012, 22, 15183 RSC.
- G. Zhu, Y. Shi, M. Mikami, Y. Shimomura and Y. Wang, CrystEngComm, 2014, 16, 6089 RSC.
- G. C. Mather, S. García-Martín, D. Benne, C. Ritter and U. Amador, J. Mater. Chem., 2011, 21, 5764 RSC.
- P. R. Slater, D. P. Fagg and J. T. S. Irvine, J. Mater. Chem., 1997, 7, 2495 RSC.
- X. Yu, T. Wang, X. Xu, T. Jiang, H. Yu, Q. Jiao, D. Zhou and J. Qiu, RSC Adv., 2014, 4, 963 RSC.
- F. Clabau, X. Rocquefelte, T. Le Mercier, P. Deniard, S. Jobic and M. H. Whangbo, Chem. Mater., 2006, 18, 3212 CrossRef CAS.
- W. Zeng, Y. H. Wang, S. C. Han, W. B. Chen, G. Li, Y. Z. Wang and Y. Wen, J. Mater. Chem. C, 2013, 1, 3004 RSC.
- R. Chen, J. Appl. Phys., 1969, 40, 570 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |