TiO2 sol-embedded in electroless Ni–P coating: a novel approach for an ultra-sensitive sorbitol sensor†
Pranee Rattanawaleedirojn*a,
Kanokwan Saengkiettiyuta,
Yuttanant Boonyongmaneerata,
Supin Sangsukb,
Nadtinan Promphetc and
Nadnudda Rodthongkuma
aMetallurgy and Materials Science Research Institute, Chulalongkorn University, Soi Chula 12, Phayathai Road, Pathumwan, Bangkok 10330, Thailand. E-mail: pranee.r@chula.ac.th
bSchool of Agricultural Resources, Chulalongkorn University, Soi Chula 64, Phayathai Road, Pathumwan, Bangkok 10330, Thailand
cNanoscience and Technology Program, Graduate School, Chulalongkorn University, Bangkok 10330, Thailand
First published on 4th July 2016
Abstract
A Ni–P–TiO2 coating was readily prepared by direct incorporation of nano-TiO2 sol into a Ni–P solution followed by electroless deposition. This coating was applied as a working electrode in an electrochemical sensor for the first time. The morphologies of the TiO2 sol and the coated surface were well characterized by TEM, SEM and AFM. The high hydrophilicity of this surface was verified by contact angles of 40.7/41.8. Here, the appropriate amount of TiO2 within the nanocomposite was optimized (2 g L−1) prior to applying as an electrode. Interestingly, the electrocatalytic activity of the coating towards the oxidation of alcoholic compounds was investigated by linear sweep voltammetry. Apparently, incorporation of TiO2 into the composites substantially improved the electrocatalytic activity of Ni–P and 2 layers of Ni–P/Ni–P–TiO2 coating provided the highest sensitivity for all analytes, especially for sorbitol. A low LOD value of 1.0 nM and a wide linear range of 2.0 nM to 0.2 mM were achieved for sorbitol. Furthermore, a high stability and high reproducibility (2.96% RSD) for this system were obtained. Owing to ultra-high sensitivity, wide linearity, high stability, easy preparation and low cost, it might be a promising tool for early diagnosis of diabetes via sorbitol detection.
1 Introduction
Electroless plating of Ni–P, readily produced by a redox reaction between nickel ion and a phosphorus-containing reducing agent, is one of the most promising surfaces widely applied in various industries, such as chemical, automotive as well as electronics.1,2 This is mainly due to its excellent mechanical and corrosion resistant properties.3–5 To further improve Ni–P coating properties and create entirely new features to the surface for expanding its applicability, embedding of nanomaterials into a Ni–P coated surface becomes a very attractive approach.6–10Among nanomaterials, incorporating of TiO2 nanoparticles to Ni–P matrix has attracted tremendous interest in scientific researches owing to its outstanding properties including hardness, wear resistance11,12 antimicrobial,13 photocatalytic14 and electrocatalytic property.15–17 Recently, Ni and Ni–P coated steel surfaces, have been used as working electrodes in electrochemical sensor for the detection of ethanol,16,18 methanol, formaldehyde and glucose.17 Incorporating of TiO2 into electroless Ni–P coating substantially enhance its electrocatalytic activity in the detection of such compounds, leading to significantly improved sensitivity of the sensors.16,17 Nonetheless, self-agglomeration of TiO2 is unavoidable since the high surface energy of nanomaterials usually leads to poor dispersion and low homogeneity on the coated surface. Lately, sol–gel enhanced electroless plating of Ni–P was developed to prepare the highly dispersive TiO2 nanoparticles within Ni–P composite coating, allowing for the increase of TiO2 content in the composites.19 Interestingly, TiO2 sol prepared in this study offers outstanding advantages including fast and simple preparation process20 and provides high stability of TiO2 sol. Particularly, TiO2 sol prepared by this approach can be directly mixed with Ni–P solution in an initial step prior to electroless plating without any agglomeration, making this process much simpler than the previous reports.
However, little attention has been paid to the in situ incorporation of TiO2 sol in Ni–P matrix,19,21 so far there is no previous report on the application of Ni–P–TiO2 based electrodes prepared by sol–gel for electrochemical sensor. Due to the high uniformity, high homogeneity, high hydrophilicity and excellent electrocatalytic property of Ni–P–TiO2 coated surface developed by our approach, it opens a new pathway for an electrochemical sensor application.
Sorbitol, increasing by polyol pathway flux in human body was recently reported as one of the crucial biomarkers for diabetic neuropathy.22–25 Traditional methods, such as high performance liquid chromatography (HPLC),23,24 spectrophotometry and enzyme based assay are currently used for sorbitol detection;26 however, these methods still have some limitations including high cost, tedious and time consuming, specialist requirement and non-portability, which limit for field-based applications. Hence, electrochemical detection becomes an alternative tool for sorbitol sensor due to its simplicity, low cost, rapid analysis, portability and simultaneously providing both qualitative and quantitative information.
Here, we develop Ni–P–TiO2 based electrodes using electroless plating of Ni–P coupled with sol–gel method and apply them as the working electrodes for sensitive detection of alcoholic compounds, especially sorbitol for the first time. The well-defined surface morphologies and hydrophilicity of Ni–P–TiO2 based coatings are investigated. Ultimately, the analytical performances of Ni–P/Ni–P–TiO2 electrode are thoroughly optimized for sorbitol. This sensing system might be an alternative tool for early diagnosis of diabetes via sorbitol detection.
2 Experimental
2.1. Materials and reagents
Titanium diisopropoxide bis(acetylacetonate) or TIAA (75 wt% in isopropanol) was purchased from Sigma-Aldrich. 1,3-Propanediol (98 wt%) was obtained from ACROS organic. Lactic acid (88 wt%) was purchased from Lobachemie. Tri-sodium citrate dehydrate, ammonium sulphate, nickel(II) sulphate, sodium hypophosphite, methanol, ethanol and hydrochloric acid were obtained from Carlo Erba reagent. Sodium hydroxide pellets, isopropanol, sorbitol, glucose and thiourea were purchased from Ajax Finechem. All chemicals were used as their purchased forms.2.2. Preparation of TiO2 sol, Ni–P and Ni–P–TiO2 coated steel surfaces
| Bath composition | Electroless plating condition | ||
|---|---|---|---|
| 1 layer Ni–P–TiO2 coating | 2 layers Ni–P/Ni–P–TiO2 coating | ||
| Basal layer | Top layer | ||
| NiSO4·6H2O | 39.5 g L−1 | 39.5 g L−1 | 39.5 g L−1 |
| Lactic acid | 40.5 g L−1 | — | 40.5 g L−1 |
| Na3C6H5O7·2H2O | — | 35 g L−1 | — |
| (NH4)2SO4 | 30 g L−1 | 30 g L−1 | 30 g L−1 |
| NaH2PO2·H2O | 20 g L−1 | 20 g L−1 | 20 g L−1 |
| Thiourea | 0.8 ppm | 0.8 ppm | 0.8 ppm |
| TiO2 | 1, 2, 4 g L−1 | — | 2 g L−1 |
| pH | 6.5 | 9.5 | 6.5 |
| Temperature | 80 ± 2 °C | 80 ± 2 °C | 80 ± 2 °C |
2.3. Material and coated surface characterization
The morphology of TiO2 sol was investigated by using a JEM-2100 transmission electron microscope (Japan Electron Optics Laboratory, Japan). The crystal structure of TiO2 sol after calcination at 600 °C for 1 hour was characterized by X-ray diffractometer (Rigaku, SmartLab) with scan rate of 10–80 degree and scan speed of 1 degree per min. The morphology of Ni–P and Ni–P–TiO2 coated steel surfaces was examined by JSM-6400 scanning electron microscope (Japan Electron Optics Laboratory, Japan). The surface area (S) and surface roughness (Ra) of the coatings were studied by using SPA-400 atomic force microscope (Seiko Instruments, Inc., Japan) using non-contact mode over a screen area of 5 × 5 μm2. The contact angle measurements of bare steel, Ni–P and Ni–P–TiO2 coated steel surfaces were performed on a 200-k1 goniometer (Rame-hart Instrument, USA) using a static sessile drop method at 5 different positions on each surface.2.4. Electrocatalytic oxidation study
The electrocatalytic oxidation studies of each electrode towards the alcoholic compounds were performed on a CHI 1240B electrochemical analyzer (CH Instruments, USA). Linear sweep voltammetric measurements were carried out using a three electrode system, consisting of Pt wire as a counter electrode, Ag/AgCl as a reference electrode and the prepared Ni alloys coated steels as the working electrodes. An in-house electrochemical cell described in the previous report was employed for all electrochemical experiments.28 The applied potential was scanned from 0.2 to 1.0 V at a scan rate of 50 mV s−1. After adding 1700 μl of 0.5 M NaOH (a supporting electrolyte) and 300 μl of each analyte solution (methanol, ethanol, isopropanol, 0.1 M glucose, 0.1 M sorbitol) into an electrochemical cell with a solution contacted surface area of 0.1256 cm2, then the electrochemical performances of modified electrodes were measured. The configuration of an electrochemical cell is illustrated in Fig. S1 in the ESI.† All electrochemical experiments were performed at room temperature of 28 ± 2 °C with at least 3 replicates. All solutions employed for electrocatalytic studies were freshly prepared by using MilliQ water type II (12.8 MΩ cm−1).3 Results and discussion
3.1. Characterization of nanosized TiO2 prepared by sol–gel method
In this study, the preparation of nanosized TiO2 particles by sol–gel method was accomplished and these particles were successfully incorporated into Ni–P solution at the beginning prior to electroless plating process. To identify the crystal structure of TiO2 sol, XRD experiment was performed on TiO2 powder after calcination at 600 °C for 1 h as shown in Fig. S2 in the ESI.†Initially, the morphology of synthesized TiO2 sol was characterized by TEM and the obtained results are shown in Fig. 1. This TEM image confirmed that the as-prepared TiO2 nanoparticles are spherical and high uniformity in shape with the average size in a range of 50–170 nm. Moreover, TiO2 nanoparticles in isopropanol shows well dispersion without any agglomeration (Fig. 1-top). The presence of TiO2 nanoparticles in isopropanol was verified by TEM-EDX analysis with the characteristic peaks of Ti as shown in Fig. 1-bottom.
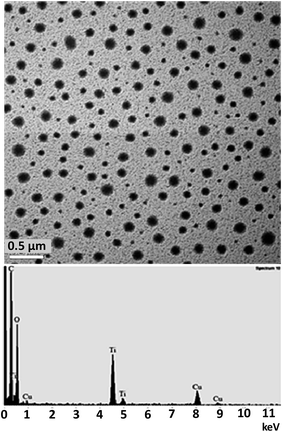 | ||
| Fig. 1 A TEM image (top) and an EDX spectrum (bottom) of the as-prepared TiO2 sol (1 M) dispersed in isopropanol. | ||
Then the as-prepared TiO2 nanoparticles were directly added into electroless Ni–P bath and it shows the high homogeneity verifying that TiO2 sol was successfully incorporated into Ni–P solution as shown in Fig. S3 in the ESI.† This simple step of preparation with well dispersion of TiO2 allow for the preparation of Ni–P–TiO2 solution at a concentration of TiO2 as high as 4 g L−1. After that, this Ni–P–TiO2 solutions were used for all electroless plating experiment.
3.2. Characterization of Ni–P and Ni–P–TiO2 nanocomposite coated surfaces
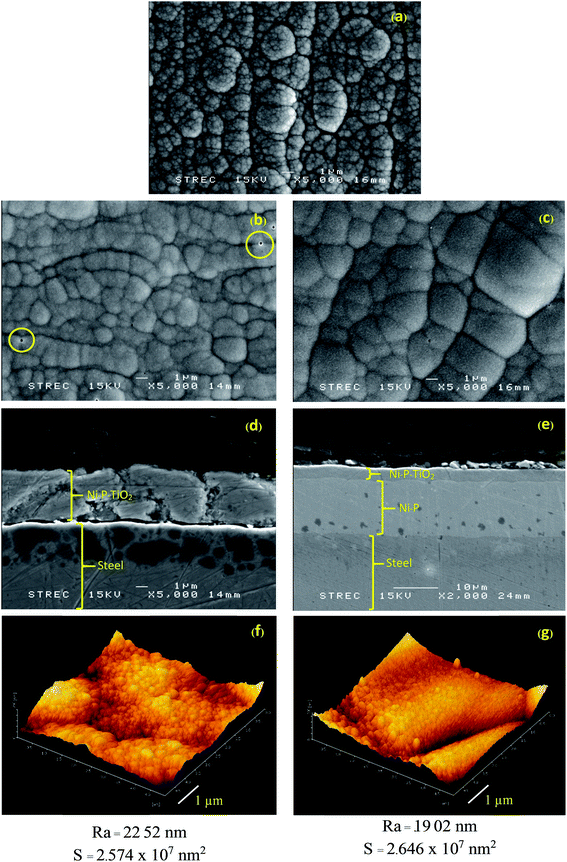
The as prepared Ni–P and Ni–P–TiO2 solutions were used for electroless plating on the pretreated steel surface to improve the surface properties. Then, the surface appearances and morphologies of coated steels were investigated by SEM. As for the amount of TiO2 sol within the composites, different concentrations of TiO2 ranging from 1, 2 and 4 g L−1 were investigated. Unfortunately, the non-reproducible coated surfaces were obtained at low concentration of TiO2 (1 g L−1), while the random micro-pores formation tended to increase on the coated surfaces at a high concentration of TiO2 (4 g L−1). This is possibly caused by the entrapment of hydrogen gas generated by electroless plating reaction onto the non-active sites of TiO2 nanoparticles during the deposition step. In this study, 2 g L−1 of TiO2 provides the highest homogeneity of the coated surfaces with the high reproducibility of coating, thus 2 g L−1 of TiO2 was selected for all subsequent experiments.The SEM images in Fig. 2 show the microstructural morphology of Ni–P, Ni–P–TiO2 and Ni–P/Ni–P–TiO2 coated steel surfaces, respectively. In general, Ni–P coated surface (Fig. 2a) shows protrude nodular structure consisting of small grains, whereas Ni–P–TiO2 coated surface (Fig. 2b) exhibits smoother nodular structure. As seen in Fig. 2b, there are some pores on the surface, leading to the crack initiation and delamination of the deposited surface as illustrated in its cross-sectional image (Fig. 2d). Thus, this defect was minimized by developing of 2 layers Ni–P/Ni–P–TiO2 coated surface as shown in Fig. 2c, revealing less pores, smoother surface and larger nodules compared with 1 layer Ni–P–TiO2 layer deposition and the less crack of this coated surface is shown in its cross-sectional image (Fig. 2e). AFM images of 1 layer Ni–P–TiO2 and 2 layers Ni–P/Ni–P–TiO2 with the same amount of TiO2 (2 g L−1) indicate that number of coating layer has no significant effect on the surface area and the surface roughness of the coated surfaces (Fig. 2f and g). An effect of different concentration of TiO2 in the electroless bath on the surface area and the surface roughness of the coated surface was also examined by AFM as shown in Fig. S4 in the ESI.† To obtain 2 layers Ni–P/Ni–P–TiO2 coating, an appropriate type of complexing agent in electroless deposition was selected for each layer. For Ni–P basal layer, trisodium citrate was used as a complexing agent by plating for 60 min since it contained high percentage of Ni (Table 2), leading to high hardness of coated surfaces.29 In contrast, lactic acid was chosen as a complexing agent by 20 min plating for Ni–P–TiO2 top layer coating, which is responsible for electrocatalytic oxidation, because it is more compatible with TiO2 sol prepared by using our protocol. The elemental composition of the coated surfaces obtained from SEM-EDX analyses is shown in Table 2. Moreover, the distribution of TiO2 with fulled coverage on the Ni–P/Ni–P–TiO2 coated steel surface was verified by SEM-EDX using a surface mapping mode as shown in Fig. S5 in the ESI.†
| Coating layer | Elemental composition (wt%) | ||||
|---|---|---|---|---|---|
| Ni | P | Ti | Fe | ||
| Ni–P | 80.6 | 16.9 | — | 2.5 | |
| Ni–P–TiO2 | 77.1 | 18.5 | 1.5 | 2.9 | |
| Ni–P/Ni–P–TiO2 | Ni–P (basal) | 95.8 | 3.8 | — | 0.4 |
| Ni–P–TiO2 (top) | 80.0 | 18.5 | 1.3 | 0.2 | |
Prior to applying these coated surfaces as the working electrodes for electrochemical sensor, the hydrophilicity of the coated surfaces was investigated by contact angle (CA) measurement. Generally, when the CA value is greater than 90°, the surface is hydrophobic and the surface becomes hydrophilic when this value is less than 90°. In this work, the water drop images and the average contact angle values of Ni–P/Ni–P–TiO2 coated steel compared with Ni–P coating and bare steel are shown in Fig. 3 and Table 3, respectively. The water contact angle on Ni–P coated surface is smaller than the one on uncoated steel, however it is higher than 90° implying the hydrophobicity of both surfaces. On the other hand, the significant decreasing of the contact angle to 41° led to spreading of water drop onto the Ni–P/Ni–P–TiO2 coated surface (Fig. 3c) revealing the high hydrophilicity of the surface mainly caused by the incorporation of TiO2 sol into the top layer. This is possibly explained by the oxygen and hydroxyl groups of TiO2 sol exposing to the coated surfaces. Another reason promoting the high hydrophilicity of the TiO2 coated surface is probably caused by the porous structure of TiO2 prepared by sol–gel method. The high hydrophilicity promotes the aqueous analyte solution to penetrate into the active surface area of electrode, leading to an improved detection sensitivity of the sensor.
 | ||
| Fig. 3 Water drop images of (a) bare steel, (b) Ni–P coated steel and (c) Ni–P/Ni–P–TiO2 coated steel. | ||
| Surface | Contact angle (°) (left/right) |
|---|---|
| Bare steel | 109.2/107.6 |
| Ni–P coated steel | 94.2/93.8 |
| Ni–P/Ni–P–TiO2 coated steel | 40.7/41.8 |
Furthermore, an influence of TiO2 concentration in an electroless bath on the hydrophilicity of the coated surface was investigated to prove that TiO2 is occupied at the outer surface of the coating and TiO2 is the main factor controlling the surface wettability. As shown in Fig. 4, the average contact angle of the coated surface substantially decreases upon the increase of TiO2 concentration.
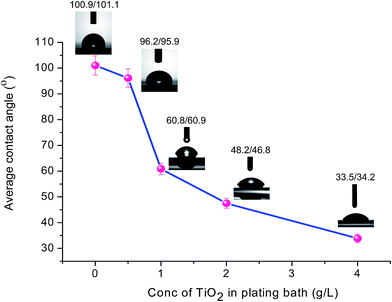 | ||
| Fig. 4 The relationship between concentration of TiO2 in electroless plating bath and the average contact angle value of the coated surfaces. | ||
According to the microstructural analyses, elemental composition, and especially CA results, they indicate the high potential of applying this Ni–P/Ni–P–TiO2 coated surface as the working electrodes in electrochemical sensor.
3.3. Electro-oxidation of alcoholic compounds on Ni–P and Ni–P–TiO2 electrodes
In this study, the electrocatalytic activity of the as prepared Ni–P and Ni–P–TiO2 coated steel electrodes was initially investigated towards the oxidation of five alcoholic compounds, including methanol, ethanol, isopropanol, sorbitol and glucose in 0.5 M NaOH by using linear sweep voltammetry (LSV). The linear sweep voltammograms of all analytes measured on bare steel, Ni–P coated steel and Ni–P–TiO2 coated steel electrodes are shown in Fig. 5.When the bare steel was used as a working electrode, there is no oxidation peak of analytes (black line). Obviously, the oxidation peaks of all analytes start showing up when Ni–P coated steel was used as a working electrode (red line). The increase of this oxidation current on Ni–P electrode is corresponded with the formation of NiOOH due to the oxidation reaction of Ni2+ in an alkaline solution explained by the reactions of (1) and (2).17 When Ni–P or Ni–P–TiO2 coated steel were used as the working electrodes, the oxidation currents of all analytes start increasing at a potential range of 0.3–0.5 V (vs. Ag/AgCl) and the oxidation peak currents were obtained at the potentials of 0.80 V, 0.66 V, 0.81 V, 0.70 V and 0.85 V for methanol, ethanol, isopropanol, sorbitol and glucose, respectively. These results correspond well with the previous reports that Ni based electrodes showed the electrocatalytic activity toward the oxidation of ethanol,16,18 methanol and glucose in an alkaline solution.17
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− | (1) |
| NiOOH + R–OH ↔ Ni(OH)2 + products | (2) |
Overall, the incorporation of TiO2 into Ni–P coated surfaces seems to further improve the electrocatalytic oxidation of all analytes. In this study, by varying the amount of TiO2 within Ni–P–TiO2 composites, the highest oxidation currents for all analytes were obtained on Ni–P–TiO2 electrode with an optimum concentration of TiO2 at 2 g L−1. At the higher TiO2 concentration (i.e. 4 g L−1), the lower oxidation current was observed, which was possibly caused by an excess amount of low conductive TiO2 on the electrode surface. Thus, compromising between the conductivity and electrocatalytic property of Ni–P–TiO2 electrode is required to be optimized. Moreover, the calculated current densities of all analytes measured on different working electrodes are shown in Fig. 6 and Table 4, respectively. These results show the highest electrochemical sensitivity of Ni–P–TiO2 (2 g L−1 TiO2) electrode toward the oxidation of sorbitol, which the oxidation current was 12 times higher than the current measured on Ni–P electrode. As explained in Section 3.2., this optimum amount of TiO2 (2 g L−1) also provides the good coverage with homogeneous coated surface, supporting the high electrochemical sensitivity. Thus, it is very interesting to apply this coated surface as a working electrode in electrochemical sensor, especially for sensitive detection of sorbitol.
 | ||
| Fig. 6 Comparison of the calculated oxidation current density of all analytes measured on Ni–P and Ni–P–TiO2 electrodes. | ||
| Electrode | Oxidation current density (mA cm−2) | ||||
|---|---|---|---|---|---|
| Methanol | Ethanol | Isopropanol | Sorbitol | Glucose | |
| Ni–P | 3.57 | 8.73 | 6.09 | 2.77 | 9.28 |
| Ni–P–TiO2 (1 g L−1 TiO2) | 7.14 | 11.81 | 10.77 | 13.85 | 9.95 |
| Ni–P–TiO2 (2 g L−1 TiO2) | 15.37 | 20.55 | 20.61 | 33.23 | 13.27 |
| Ni–P–TiO2 (4 g L−1 TiO2) | 8.79 | 19.52 | 12.65 | 22.15 | 11.94 |
3.4. Improvement of the electrocatalytic oxidation of sorbitol by using 2 layers Ni–P/Ni–P–TiO2 coating
As shown in the Section 3.2., 2 layers coating of Ni–P/Ni–P–TiO2 shows less pore and smoother surface compared to 1 layer Ni–P–TiO2 coating. In order to select the most suitable coating surface for the electrochemical sensor, the electrochemical performances of these Ni–P–TiO2 based coatings were evaluated by the oxidation reaction of sorbitol in 0.5 M of NaOH. As illustrated in Fig. 7, the higher oxidation current signal (1.4 times) of sorbitol measured on 2 layers Ni–P/Ni–P–TiO2 coating compared with 1 layer Ni–P–TiO2 coating verified the substantially improved electrochemical sensitivity of Ni–P/Ni–P–TiO2 electrode for this sensing system. This could be possibly explained by the well coverage without defect of the coating, which might help to facilitate the electron transfer between the electrode surface and the analyte solution. Furthermore, it could be noticed that % RSD (the error bars of Fig. 7b) of 2 layers Ni–P/Ni–P–TiO2 coating (2.3%) is 3 times lower than the one for 1 layer Ni–P–TiO2 coating (7.2%) verifying the higher coating reproducibility of 2 layers Ni–P/Ni–P–TiO2 coated surfaces.In addition, the stability of these electrodes was investigated compared with Ni–P electrode as shown in Table 5. After 7 days of storage at a room temperature (28 ± 2 °C) with 10 replicates of measurement, % of current response signal compared to the original signal was highest on 2 layers Ni–P/Ni–P–TiO2 coated electrode. The current responses remained 96.4% of their initial values with 1.1% RSD, verifying the high stability of this electrode. Also, the stabilities of these electrodes toward glucose and other alcoholic compounds were studied as shown in Table S1 in the ESI.†
| Electrode | % of current signal compared to an original current signal |
|---|---|
| Ni–P | 85.0 ± 16 |
| Ni–P–TiO2 | 91.0 ± 4.1 |
| Ni–P/Ni–P–TiO2 | 96.4 ± 1.1 |
Furthermore, the reproducibility of 2 layers Ni–P/Ni–P–TiO2 modified electrodes was investigated by measuring LSV responses to 1 μM of sorbitol in 0.5 M NaOH for 10 replicates. The percentage relative standard deviation (% RSD) of the oxidation peak currents obtained by 10 successive measurements was found to be 2.96%, indicating excellent detection reproducibility as shown in Fig. S6 in the ESI.† This result suggests that this electrode was highly stable and suitable for long term of usage as a sensor.
3.5. The analytical performances of 2 layers Ni–P/Ni–P–TiO2 coated steel for sorbitol sensor
Then the applicability of 2 layers Ni–P/Ni–P–TiO2 coated steel as a working electrode for sorbitol sensor was evaluated. To investigate the analytical performances of this sensor, the relationship between the sorbitol concentration and oxidation current was studied by linear sweep voltammetry (LSV). As shown in Fig. 8, the current response increased as a function of the sorbitol concentration, and a linear range was found to be 2.0 nM to 0.2 mM with a correlation coefficient (R2) of 0.9904. The limit of detection (LOD) for the system evaluated using signal-to-noise ratios of three (S/N = 3) was found to be 1.0 nM, which was much lower than the previous reports.22,24,25,30–32 Based on the clinical report, a level of 5.1 μM is required to differentiate between normal and diabetic subjects,24 which verify that our obtained detection limit is sensitive enough for diabetes diagnosis. Owing to an ultra-low detection limit and high sensitivity, our approach might be a novel sensor for sorbitol in early stage diagnosis.To examine the selectivity of sorbitol sensor, LSV measurement was conducted by adding the same concentration of glucose (0.1 mM), another sugar alcohol which is highly possible to be contaminated in the determination of sorbitol in real samples,24,25 into 0.1 mM of sorbitol solution. As shown in Fig. 9, a peak potential of sorbitol was found to be 0.68 V, while a peak potential of glucose was 0.85 V. The separated peak currents between these two analytes confirm that Ni–P/Ni–P–TiO2 modified electrodes can selectively determine sorbitol in the presence of glucose. Especially, this sensor is selective enough for the detection of low concentration of sorbitol (5.1 μM) required in medical diagnosis.24
 | ||
| Fig. 9 The selectivity of Ni–P/Ni–P–TiO2 electrode for sorbitol detection in the presence of a same concentration of glucose (0.1 mM). | ||
Based on the obtained analytical performances of Ni–P/Ni–P–TiO2 coated steel electrode, this system is very promising for sensitive sorbitol sensor, thus it might be an alternative tool for sensitive detection of sorbitol in various fields, especially for medical diagnosis.
4 Conclusions
A high performance electrochemical sensor based on Ni–P–TiO2 electrode was successfully developed. Highly dispersive TiO2 nanoparticles incorporated into electroless Ni–P solution were simply prepared via a sol–gel method. The smooth nodular structure with a perfect coverage on steel and a hydrophilic surface of Ni–P/Ni–P–TiO2 dictated its potential application for electrochemical sensor. By using a prepared Ni–P/Ni–P–TiO2 as a working electrode for electrocatalytic oxidation of alcoholic compounds, TiO2 containing Ni–P coating showed higher electrocatalytic property compared with Ni–P coating and bare steel, particularly for sorbitol detection. This system exhibited an ultra-high sensitivity with a low LOD of 1.0 nM, a wide linear range of 2.0 nM to 0.2 mM, high stability, high reproducibility and high selectivity towards sorbitol detection.Acknowledgements
This research has been supported by the Ratchadaphiseksomphot Endowment Fund 2013 of Chulalongkorn University (CU-56-494-AM).References
- G. O. Mallory and J. B. Hajdu, Electroless Plating: Fundamentals and applications, American Electroplaters and Surface Finishers Society, New York, 1990 Search PubMed.
- M. Schlesinger, Electroless Deposition of Nickel, in Modern Electroplating, ed. M. Schlesinger and M. Paunovic, John Wiley & Sons, New York, 2010 Search PubMed.
- M. Yan, H. G. Ying and T. Y. Ma, Surf. Coat. Technol., 2008, 202, 5909–5913 CrossRef CAS.
- R. Rajendran, W. Sha and R. Elansezhian, Surf. Coat. Technol., 2010, 205, 766–772 CrossRef CAS.
- M. Islam, M. R. Azhar, N. Fredj and T. D. Burleigh, Surf. Coat. Technol., 2013, 236, 262–268 CrossRef CAS.
- J. N. Balaraju, T. S. N. S. Narayanan and S. K. Seshadri, Mater. Res. Bull., 2006, 41, 847–860 CrossRef CAS.
- T. Rabizadeh and S. R. Allahkaram, Mater. Des., 2011, 32, 133–138 CrossRef CAS.
- C. K. Lee, Int. J. Electrochem. Sci., 2012, 7, 12941–12954 CAS.
- S. Ranganatha, T. V. Venkatesha and K. Vathsala, Appl. Surf. Sci., 2012, 263, 149–156 CrossRef CAS.
- Z. Sharifalhoseini and M. H. Entezari, Appl. Surf. Sci., 2015, 351, 1060–1068 CrossRef CAS.
- P. Makkar, R. C. Agarwala and V. Agarwala, Ceram. Int., 2013, 39, 9003–9008 CrossRef CAS.
- P. Makkar, R. C. Agarwala and V. Agarwala, Adv. Powder Technol., 2014, 25, 1653–1660 CrossRef CAS.
- Q. Zhao, C. Liu, X. Su, S. Zhang, W. Song, S. Wang, G. Ning, J. Ye, Y. Lin and W. Gong, Appl. Surf. Sci., 2013, 274, 101–104 CrossRef CAS.
- L. Xiong, G. Q. Zhang and H. G. Pan, Adv. Mater. Res., 2011, 311–313, 319–322 CrossRef CAS.
- S. M. A. Shibli and V. S. Dilimon, Int. J. Hydrogen Energy, 2007, 32, 1694–1700 CrossRef CAS.
- S. M. A. Shibli, N. D. Suma and V. S. Dilimon, Sens. Actuators, B, 2008, 129, 139–145 CrossRef CAS.
- A. Abdel Aal, H. B. Hassan and M. A. Abdel Rahim, J. Electroanal. Chem., 2008, 619–620, 17–25 CrossRef CAS.
- M. Wan, Z. Zhuang, J. Dai and D. Xiao, Russ. J. Electrochem., 2011, 47, 96–101 CrossRef CAS.
- W. Chen, W. Gao and Y. He, Surf. Coat. Technol., 2010, 204, 2493–2498 CrossRef CAS.
- S. Tangwiwat and S. J. Milne, J. Sol-Gel Sci. Technol., 2005, 34, 147–154 CrossRef CAS.
- S. Ranganatha, T. V. Venkatesha and K. Vathsala, Appl. Surf. Sci., 2010, 256, 7377–7383 CrossRef CAS.
- T. Gessei, T. Arakawa, H. Kudo and K. Mitsubayashi, Analyst, 2015, 140, 6335–6342 RSC.
- H. J. Sim, J. S. Jeong, H. J. Kwon, T. H. Kang, H. M. Park, Y. M. Lee, S. Y. Kim and S. P. Hong, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 1607–1611 CrossRef CAS PubMed.
- S. Kwang-Hyok, P. Ui-Nam, C. Sarkar and R. Bhadra, Clin. Chim. Acta, 2005, 354, 41–47 CrossRef CAS PubMed.
- H. P. Anaja, Clin. Chim. Acta, 1997, 262, 1–11 CrossRef CAS.
- R. Shinohara, Y. Ohta, M. Yamauchi and I. Ishiguro, Clin. Chim. Acta, 1998, 273, 171–184 CrossRef CAS.
- K. Theeratatpong, S. Danchaivijit and Y. Boonyongmaneerat, J. Met., Mater. Miner., 2013, 23, 75–79 CAS.
- N. Rodthongkum, N. Ruecha, R. Rangkupan, R. W. Vachet and O. Chailapakul, Anal. Chim. Acta, 2013, 804, 84–91 CrossRef CAS PubMed.
- K. G. Keong, W. Sha and S. Malinov, Surf. Coat. Technol., 2003, 168, 263–274 CrossRef CAS.
- J. Vetrommen, E. Rillaerts, M. Gyseles and I. Deleew, Diabetes Metab., 1987, 13, 182–186 Search PubMed.
- J. I. Malone, G. Knox, S. Benford and T. A. Tedesco, Diabetes Care, 1980, 29, 861–864 CrossRef CAS.
- F. Renner, A. Schimtz and H. Gehring, Clin. Chem., 1998, 44, 886–888 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05090e |
| This journal is © The Royal Society of Chemistry 2016 |