Identification of novel thermostable ω-transaminase and its application for enzymatic synthesis of chiral amines at high temperature†
Sam Mathewa,
Kanagavel Deepankumarb,
Giyoung Shinc,
Eun Young Hongd,
Byung-Gee Kimd,
Taeowan Chunge and
Hyungdon Yun*a
aDepartment of Bioscience & Biotechnology, Konkuk University, Seoul, South Korea. E-mail: hyungdon@konkuk.ac.kr
bSchool of Materials Science and Engineering, Biological & Biomimetic Material Laboratory, Nanyang Technological University, Singapore
cSchool of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology, Pohang, Gyeongbuk, Republic of Korea
dSchool of Chemical and Biological Engineering, Seoul National University, Seoul, South Korea
eSchool of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk, South Korea
First published on 13th July 2016
Abstract
A novel thermostable ω-transaminase from Thermomicrobium roseum which showed broad substrate specificity and high enantioselectivity was identified, expressed and biochemically characterized. The advantage of this enzyme to remove volatile inhibitory by-products was demonstrated by performing asymmetric synthesis and kinetic resolution at high temperature.
Enantiomerically pure amines are frequently used as precursors in pharmaceutical drugs, fine chemicals and many other natural products.1 Among the various enzymatic routes to synthesize amines, ω-transaminases (ω-TA) has emerged as a potent class of enzyme to generate a wide range of optically pure amines and unnatural amino acids.2 In recent years, several cascade reactions using ω-TAs and co-enzymes were developed to efficiently produce chiral amine compounds from various classes of substrates.3 Similarly, over the years, several ω-TA mutants were generated to enhance the substrate scope and reactivity of wild-type ω-TAs. One notable example is the development of a mutant (R)-ω-TA from Arthrobacter sp. for synthesizing sitagliptin, an active compound of the drug-Januvia.4
Despite many advancements in synthesizing amines using ω-TAs, the number of ω-TAs used in industries remain rather modest. Good operational stability in the presence of high temperature, organic solvents and other substrates can make ω-TAs ideal for industrial applications. Directed evolution method was earlier utilized to enhance the activity and thermostability of ω-TA from Athrobacter citreus which resulted in lower enzyme loading to synthesize (S)-tetraline.5 Recently, the thermostability and organic solvent tolerance of ω-TA from Vibrio fluvialis (ω-TAVF) was enhanced by residue specific incorporation of fluorotyrosine.6 Similarly, fluoroproline incorporation was used to enhance the stability and site-specific immobilization of ω-TA from Sphaerobacter thermophilus.7 However, incorporation of unnatural amino acids into enzymes can be expensive and may not be suitable for industrial applications. Another route to identify highly stable enzymes is by sourcing it from thermophiles. Thermophiles which have an optimal growth above 60 °C constitute an excellent source for identifying thermostable ω-TAs. To the best of our knowledge, except for the recently reported ω-TA from Sphaerobacter thermophiles and taurine–pyruvate TA from Geobacillus thermodenitrificans, all other ω-TAs that are reported to date are from non-thermophilic organisms which are often incapable to operate at elevated temperatures.8,9 With the advent of computational methods, it is possible to screen enzymes of our interest from protein sequence databases. Hohne et al. had identified novel (R)-ω-TAs by developing an in silico strategy for a sequence-based prediction of substrate specificity and enantio-preference.10 A similar sequence based strategy was recently utilized to identify 6 more (R)-ω-TAs.11 An alternate simple way to identify enzymes of our interest is by performing a BLAST search, provided we have a suitable enzyme as our query sequence and the resulting hits have good identity with the query sequence (>35%).2e
To identify a novel thermostable (S)-ω-TA, a BLAST search was performed against protein sequences from thermophiles using ω-TA from Vibrio fluvialis (ω-TAVF) as query sequence. Based on the BLAST score (41% identity), aminotransferase from Thermomicrobium roseum (ω-TATR) was selected (NCBI-ID = WP_015922033.1). Thermomicrobium roseum belongs to highly thermophilic Gram positive bacteria. Previously, thermostable enzymes such as tyrosinase and alcohol dehydrogenase were sourced from this organism.12,13 The ω-TATR gene was cloned into a pET24ma vector with C-terminal His6-tag, effectively expressed in BL21 cell (DE3) and subsequently purified on a Ni-NTA affinity column (ESI, Fig. S1†). Next, to check TA activity, the purified enzyme was subjected to a typical ω-TA assay containing 20 mM (S)-α-MBA and 20 mM pyruvate in 100 mM Tris buffer (pH 7.5) at 37 °C for 30 minutes. ω-TATR showed a specific activity of 0.6 U mg−1. In this study, the above mentioned ω-TA assay was used for all enzymatic studies unless said otherwise. For determining the kinetic parameters of the enzyme, (S)-α-MBA and pyruvate were utilized for the analysis, as these substrates are considered as model substrates for ω-TA reaction (ESI, Fig. S2 and S3†). At 60 °C, the km and kcat values of ω-TATR towards (S)-α-MBA in the presence of 10 mM pyruvate were 11.7 mM and 43.4 min−1 respectively. In the case of pyruvate, the km and kcat values of ω-TATR in the presence of 10 mM (S)-α-MBA were 2.0 mM and 31.9 min−1 respectively. The kcat of ω-TATR towards pyruvate and (S)-α-MBA at 60 °C were ∼2 and ∼3 fold higher when compared to that at 37 °C.
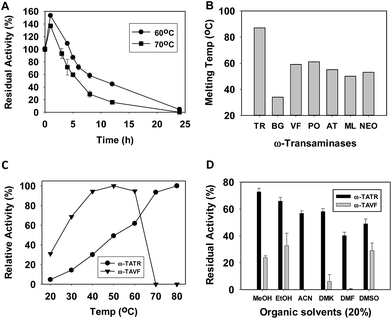
To examine the stability of the enzyme at high temperature, ω-TATR was incubated at 60 °C and 70 °C for different intervals of time and its residual activity were measured (Fig. 1A). Interestingly, the enzyme had a higher activity when its initial rate was measured after 1 h incubation. This may be due to the refolding of the enzyme to its natural conformation when exposed to high temperature. At 60 °C, ω-TATR retained ∼60% of its activity after 8 h while at 70 °C the enzyme retained similar activity after 5 h. For comparative stability study, ω-TA from Vibrio fluvialis (ω-TAVF) which is well reported was utilized. The stability of ω-TATR and ω-TAVF was investigated after incubating the enzymes at 60 °C for 2 h in the presence of different additives (ESI, Fig. S4†). The residual activity of the enzymes in the presence of 25 mM (S)-α-MBA, 25 mM pyruvate, 0.1 mM PLP and in the absence of any substrates were analysed. ω-TAVF completely lost its activity in all the cases while in the case of ω-TATR, there was considerable loss of activity only in the presence of (S)-α-MBA which may be due to the formation of E-PMP. As expected, ω-TATR showed highest initial activity in the presence of PLP as it acts as stabilizing agent by forming an E-PLP complex.14 As explained earlier, the high residual activity of ω-TATR at 2 h in the presence of PLP may be due to the better conformation of the enzyme at higher temperature. The stability of ω-TATR was also established by measuring its melting temperature with other reported ω-TAs (Fig. 1B; TR, BG, VF, PO, AT, ML and NEO represents TAs from Thermomicrobium roseum, Burkholderia graminis, Vibrio fluvialis, Polaramonas, Agrobacterium tumefaciens, Mycobacterium loti and Neosartorya fischeri), (ESI, Fig. S5†). The melting temperatures of ω-TAs were measured as explained elsewhere.15 The ω-TAVF had a melting temperature of 59 °C while ω-TATR had 87 °C which is the highest reported melting temperature for any wild-type ω-TAs. Earlier, the melting temperature of ω-TAST (it had two melting temperatures, 65 °C and 74 °C) was enhanced when the enzyme was incorporated with ω-TA[(4R)-FP] (77 °C and 89 °C).7 Interestingly, ω-TA from Chromobacterium violaceum (ω-TACV) had shown a high melting point (78 °C) even though the enzyme was sourced from a mesophilic organism.16 The effect of temperature towards ω-TATR and ω-TAVF was also probed by performing reaction at different temperatures from 20 to 80 °C (Fig. 1C) which also confirmed the better stability of ω-TATR. ω-TATR also showed better residual activity in the presence of various organic solvents (20% v/v) (Fig. 1D).
To explore the substrate specificity of ω-TATR, 18 amino donors (Fig. 2) were tested. Enzyme reactions for amino donor specificity were carried out in a reaction volume of 1 mL containing 100 mM Tris–HCl buffer (pH 7.5), 20 mM amino donor, 20 mM pyruvate, 0.1 mM PLP for 30 min at 37 °C. The enzyme's activity towards a broad range of substrates indicates its potential in producing amines. In general, among the tested substrates, ω-TATR had better activity towards aromatic amines. The poor activity of ω-TATR towards aromatic amines such as 10 and 12 could be due to the steric hindrance observed in the small binding pocket when the methyl group is substituted by bulkier functional groups. Earlier ω-TAVF and ω-TACV mutants were developed that showed better activity towards compounds such as 10 with slightly bulky side chains.17,18 Next, the amino acceptor specificity of ω-TATR was investigated towards 15 compounds (ESI, Fig. S6†). Among the tested amino acceptors, ω-TATR showed highest activity towards 2-oxo-3-phenylpropanoic acid which was ∼2 fold higher than that of pyruvate (kinetic parameters of ω-TATR were mentioned earlier). ω-TATR had also shown considerable activity towards 3-fluoro-2-oxopropanoic acid and 3-methyl-2-oxobutanoic acid which can be used to generate valuable unnatural amino acids such as 2-amino-3-fluoropropanoic acid and 2-amino-3-methylbutanoic acid by performing reductive amination of the substrates.
 | ||
| Fig. 2 (A) Amino donor spectrum. (B) Amino donor specificity of ω-TATR (enzyme activity towards 1 was taken as 100%). | ||
Along with thermostability, tolerance towards organic solvents is also important as 5–20% organic solvents are often utilized in the reaction mixture while performing enzymatic reactions using hydrophobic substrates. To inspect the tolerance of ω-TATR and ω-TAVF, the enzymes residual activity was determined after incubating it in the presence of different organic solvents (20% v/v) (Fig. 1D). The enzyme had highest residual activity in the presence of methanol while it was least in the presence of dimethylformamide. The activity of ω-TATR was better than ω-TAVF in all the cases. In the case of substrate inhibition and product inhibition studies, except for the inhibition by L-Ala, ω-TATR showed similar pattern compared to other ω-TAs (ESI, Fig. S7 and S8†). The enzyme lost substantial activity in the case of L-Ala (>90%).
To investigate the utility of ω-TATR as good catalyst, kinetic resolution of rac-amines was performed (Table 1). The rate of enzymatic synthesis can be increased by performing the reaction at increased temperature. This not only reduces enzyme required for the reaction but also minimizes the time needed for biotransformation. To explore the feasibility of low enzyme loading for enzymatic reactions, reactions were performed using 50 mM rac-amines and 50 mM pyruvate with 1 U ω-TATR at 37 °C and 60 °C. Since ω-TATR is (S)-stereoselective, after kinetic resolution (R)-amine is left behind. As expected, ee values of (R)-amines were much higher when reactions were performed in 60 °C compared to that of 37 °C. Among the aromatic compounds, except for 2, 5 and 10 all substrates were completely resolved with excellent enantiomeric excess (>99%). However, apart from 10, all substrates were completely resolved when 4 U of ω-TATR was added to the reaction mixture at 37 °C.
| Substrate | 60 °C reaction | 37 °C reaction | ||||
|---|---|---|---|---|---|---|
| Time (h) | Conv. (%) | eeR (%) | Time (h) | Conv. (%) | eeR (%) | |
| a Reaction conditions: reaction vol. 0.25 mL. 50 mM rac-(1–6,10–11), 50 mM pyruvate, (1 U mL−1) ω-TATR, 0.1 mM PLP, 100 mM Tris–HCl buffer (pH 7.5) at 37 °C or 60 °C.b ω-TATR (2 U mL−1) was used and reaction was performed for 18 h. | ||||||
| 1 | 2 | 50.1 | >99 | 18 | 47.3 | 87 |
| 2 | 18 | 48.5 | 94 (>99b) | 18 | 45.5 | 82 |
| 3 | 6 | 50.3 | >99 | 18 | 40.3 | 61 |
| 4 | 2 | 50.5 | >99 | 18 | 37.8 | 51 |
| 5 | 18 | 48.7 | 95 (>99b) | 18 | 34.8 | 39 |
| 6 | 2 | 50.2 | >99 | 18 | 47.5 | 90 |
| 10 | 18 | 40.3 | 61 (72b) | 18 | 28.0 | 12 |
| 11 | 2 | 50.1 | >99 | 18 | 46.3 | 85 |
To demonstrate the advantage of thermostable ω-TA over other mesophilic ω-TAs, we tried to obtain enantiomerically pure (R)-sec-butylamine through kinetic resolution utilizing ω-TATR. Acute product inhibition is one of the main drawbacks of ω-TA reaction and in this case the formation of 2-butanone severely affects enzyme activity.19 One way of removing inhibitory ketone is by introducing a biphasic system where by the inhibitory ketone from the reaction media will be transferred to the organic phase thereby overcoming by-product inhibition problem.20 But the solubility of sec-butylamine makes it difficult to selectively extract 2-butanone. Earlier, kinetic resolution of sec-butylamine was performed in reduced pressure to selectively remove 2-butanone utilizing its volatility in reduced pressure.19
In this study, initially, a 5 mL whole cell reaction containing over-expressed ω-TATR (180 mg mL−1), 200 mM sec-butylamine and 200 mM pyruvate was performed at 37 °C. However after 24 h reaction, the enantiomeric excess of (R)-sec-butylamine reached only 50.1% which is due to by-product inhibition as mentioned earlier. To circumvent the problem of inhibitory 2-butanone, we devised a strategy to remove 2-butanone by performing the reaction at high temperature (85 °C) (Fig. 3). Since ω-TATR was highly thermostable, we hypothesized a reaction at elevated temperature (85 °C) could remove 2-butanone as its boiling temperature is 79.6 °C. Another advantage of performing reaction at high temperature is that it accelerates the reaction speed. Based on this hypothesis, the reaction was performed at 85 °C. As expected, the reaction at elevated temperature gave better result, 72.8 mM (R)-sec-butylamine was produced with very high enantiomeric excess (ee > 99%) in 8 h. However, one drawback of this strategy was that along with the by-product, the substrate was also evaporated. To minimize this problem, we incubated the reaction media at 37 °C and 85 °C alternatively for 1 h. (R)-sec-Butylamine with high enantiomeric excess (ee > 99%) was achieved in 12 h, however the evaporated sec-butylamine was higher than the reaction which was performed in 85 °C (only 62.7 mM (R)-sec-butylamine was generated) which may be due to the longer reaction time. Subsequently, a 15 mL reaction was performed with identical condition (85 °C), which gave a final isolated yield was 25.2% (ee > 99%).
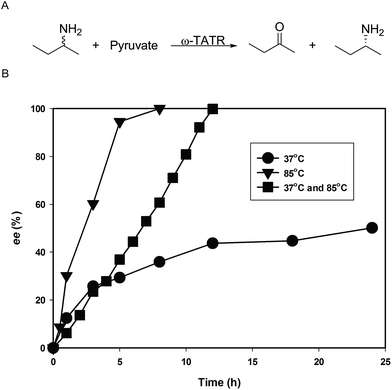 | ||
| Fig. 3 (A) Reaction scheme for the synthesis of (R)-sec-butylamine. (B) Reaction profile of sec-butylamine at different temperatures. | ||
Next, to study the application of ω-TATR via asymmetric synthesis, the amination of 10 mM acetophenone (10% DMSO) using 50 mM L-Ala as amino donor with a pyruvate removal system (LDH system; 1 mM NADH, 90 U LDH, 10 U GDH, 50 mM glucose, 1.4 U ω-TATR) was performed. Since, the enzymes used in the pyruvate removal system (lactate dehydrogenase and glucose dehydrogenase) were not thermostable, the reaction was performed in 37 °C. However, the generated (S)-α-MBA was only 16%, albeit with good optical purity (ee > 99%). Since ω-TATR was thermostable, ω-TATR (2 U mL−1) was utilized to synthesize (S)-α-MBA using 200 mM isopropylamine as amino donor. The reaction was performed at 60 °C to remove inhibitory acetone (boiling point 56 °C) which resulted in 75.6% yield in 6 h. These results clearly demonstrate the benefit of using a ω-TATR for biotransformation.
In summary, a novel thermostable ω-TA from Thermomicrobium roseum was functionally characterized and its utility in synthesizing amines was demonstrated. The enzyme's stability at very high temperature was effectively used to remove volatile by-product such as inhibitory ketones without employing any co-enzymes or by-product removal system.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea (NRF-2013R1A2A2A01068013).References
- F. Michael, J. E. Farnberger and W. Kroutil, Eur. J. Org. Chem., 2015, 6965 Search PubMed.
- (a) F. Steffen-Munsberg, C. Vickers, H. Kohls, H. Land, H. Mallin, A. Nobili, L. Skalden, T. V. D. Bergh, H. J. Joosten, P. Berglund, M. Höhne and U. T. Bornscheuer, Biotechnol. Adv., 2015, 33, 566 CrossRef CAS PubMed; (b) D. Ghislieri and N. J. Turner, Top. Catal., 2014, 57, 284 CrossRef CAS; (c) H. Kohls, F. Steffen-Munsberg and M. Höhne, Curr. Opin. Biotechnol., 2014, 19, 180 CrossRef CAS PubMed; (d) M. S. Malik, E. S. Park and J. S. Shin, Appl. Microbiol. Biotechnol., 2012, 94, 1163 CrossRef CAS PubMed; (e) S. Mathew and H. Yun, ACS Catal., 2012, 2, 993 CrossRef CAS.
- R. C. Simon, N. Richter, E. Busto and W. Kroutil, ACS Catal., 2014, 4, 129 CrossRef CAS.
- C. K. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Hmauisn and G. J. Hughes, Science, 2010, 329, 305 CrossRef CAS PubMed.
- A. R. Martin, R. Disanto, I. Plotnikov, S. Kammat, D. Shonnard and S. Pannuri, Biochem. Eng. J., 2007, 37, 246 CrossRef CAS.
- K. Deepankumar, M. Shon, S. P. Nadarajan, G. Shin, S. Mathew, N. Ayyadurai, B. G. Kim, S. H. Choi, S. H. Lee and H. Yun, Adv. Synth. Catal., 2014, 356, 993 CrossRef CAS.
- K. Deepankumar, S. P. Nadarajan, S. Mathew, S.-G. Lee, T. H. Yoo, E. Y. Hong, B. G. Kim and H. Yun, ChemCatChem, 2015, 7, 417 CrossRef CAS.
- S. Mathew, S. P. Nadarajan, T. Chung, H. H. Park and H. Yun, Enzyme Microb. Technol., 2016, 87, 52–60 CrossRef PubMed.
- Y. Chen, D. Yi, S. Jiang and D. Wei, Appl. Microbiol. Biotechnol., 2016, 100, 3101–3111 CrossRef CAS PubMed.
- M. Hohne, S. Schatzle, H. Jochens, K. Robins and U. T. Bornscheuer, Nat. Chem. Biol., 2010, 6, 807 CrossRef PubMed.
- J. Jiang, X. Chen, D. Zhang, Q. Wu and D. Zhu, Appl. Microbiol. Biotechnol., 2015, 99, 2613 CrossRef CAS PubMed.
- K. H. Kong, M. P. Hong, S. S. Choi, Y. T. Kim and S. H. Cho, Biotechnol. Appl. Biochem., 2000, 31, 113 CrossRef CAS PubMed.
- S. Y. Yoon, H. S. Noh, E. H. Kim and K. H. Kong, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, 132, 415 CrossRef.
- J. S. Shin, H. Yun, J. W. Jang, I. Park and B. G. Kim, Appl. Microbiol. Biotechnol., 2013, 61, 463 CrossRef PubMed.
- F. H. Niesen, H. Berglund and M. Vedadi, Nat. Protoc., 2007, 2, 2212–2221 CrossRef CAS PubMed.
- S. Chen, H. Land, P. Berglund and M. S. Humble, J. Mol. Catal. B: Enzym., 2016, 124, 20 CrossRef CAS.
- A. Nobili, F. Steffen-Munsberg, H. Kohls, I. Trentin, C. Schulzke, M. Höhne and U. T. Bornscheuer, ChemCatChem, 2015, 7, 757 CrossRef CAS.
- K. E. Cassimjee, M. S. Humble, V. Abedi and P. Berglund, Org. Biomol. Chem., 2012, 10, 5466 CAS.
- H. Yun, B. K. Cho and B. G. Kim, Biotechnol. Bioeng., 2004, 87, 772 CrossRef CAS PubMed.
- J. S. Shin and B. G. Kim, Biotechnol. Bioeng., 1997, 55, 48 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15110h |
| This journal is © The Royal Society of Chemistry 2016 |