Synthesis of polygonal Co3Sn2 nanostructure with enhanced magnetic properties†
Zheng Yiabc,
Xin Tianbc,
Qigang Han*ab,
Jianshe Lianc,
Yaoming Wub and
Limin Wang*b
aRoll Forging Research Institute, Jilin University, Changchun 130025, China. E-mail: hanqg@jlu.edu.cn; Fax: +86 431 85094340; Tel: +86 431 85094340
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, CAS, Changchun 130022, China. E-mail: lmwang@ciac.ac.cn; Fax: +86 431 85262836; Tel: +86 431 85262447
cCollege of Materials Science and Engineering, Jilin University, Changchun 130025, China
First published on 11th April 2016
Abstract
A one-pot solvothermal route is employed to fabricate polygonal Co3Sn2 nanostructures. The obtained product exhibits anisotropic structure and morphology, which endow the Co3Sn2 nanostructure with enhanced coercivity of 131.5 Oe, four times as high as the cubic cobalt sample (31.3 Oe).
In recent decades, metallic cobalt (Co) and Co-based intermetallics have received increasing attention due to their potential applications in very wide fields, especially as magnetic materials in magnetic resonance imaging, information storage and magnetic refrigeration, etc.1,2 Many recent approaches reveal that the functional Co-based materials can be fabricated via different routes and controlled with various shapes and structures. For instance, a variety of techniques including hydrothermal process,3 aerosol-assisted4 and template-assisted5,6 methods have been exploited for the assembly of Co-based materials. Various dimensionalities and morphologies such as zero-dimensional (0D) nanoparticles,7 1D nanowires,6,8 2D and 3D nanostructures9–11 have also been widely introduced to the developing of Co-based materials and application for the magnetic material field. In addition, several studies have focused on the Co–Sn intermetallics as anode materials in lithium-ion batteries by using the inert Co matrix to resolve the problem of rapid capacity fading over extended cycling.12–14
More and more recent investigations reveal that the shape anisotropy has a pivotal effect on the magnetic properties of metal-based materials. For example, Yao J. et al. reports that the cubic cobalt nanoskeletons exhibit enhanced coercivity in comparison with cube-like Co aggregates and cubic Co nanocages.3 Analogously, many researchers have also discovered the shape anisotropies in 1D, 2D and 3D morphologies all have pronounced influences on the magnetic properties.15–18 Apart from the shape anisotropies, numerous literatures have shown that the magnetic properties are highly dependent on the structure characteristics. As a representative example, the FeSn5 intermetallic form only a 1D network along the c-axis display drastically different magnetic properties compared with the 3D case of FeSn2 phase.19 Briefly summarize that the magnetic properties of metal-based materials intensively lie on their shape anisotropies and structure characteristics.
Metallic Sn doping is considered as a promising route to change structure characteristics of the metal-based materials, and thus improve their magnetic properties in a special application.19,20 Interestingly, during our preparation of the Co–Sn system, we find that the tin doping has not only an influence on its structure characteristics, but also an impact on its shape anisotropies. Together with the lack of magnetization data in the literature has motivated us to study the magnetic properties of the Co3Sn2 intermetallic and compare it with metallic Co alone. In our one-pot solvothermal route (see ESI†), the Sn-doped Co3Sn2 intermetallic and pure metallic Co are fabricated in a similar process but show entirely different structural and morphological characteristics. The Sn-doped Co3Sn2 intermetallic exhibits 3D polygonal morphology and anisotropic crystal structure, while the metallic Co without doping displays a 0D quasi-spherical morphology and a standard face-centred cubic (FCC) structure. In addition, based on the structural and morphological advantages, the magnetic measurement results reveal that the Sn-doped Co3Sn2 intermetallic displays the ferromagnetic behaviour with a higher coercivity compared with the Co alone. Furthermore, because of the obtained Co3Sn2 intermetallic with a higher Sn content and effective polygonal morphology also motivates us to explore its anode performances for lithium ion batteries.
One-pot solvothermal method gives the Co3Sn2 intermetallic nanostructure. Scanning electron microscope (SEM), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) provide insight into the nanostructure and morphology of the obtained product. As presented in Fig. 1a and b, the Co3Sn2 intermetallic exhibits identical polygonal morphology and relatively uniform size. A single Co3Sn2 nanostructure is shown in Fig. 1c. It can be seen that the nanostructure unit is with grain size of approximately 150 nm. The X-ray diffraction (XRD) pattern of the product is presented in Fig. 1d. All diffraction peaks of as-prepared sample agree well with the characteristic peaks of the Co3Sn2 alloy (JCPDS no. 27-1124), indicating the pure phase of the Co3Sn2 nanostructure. The HRTEM image further implies the phase structure of the Co3Sn2 intermetallic. As shown in Fig. 1e, the measured d-spacing of the selected portion is 0.29 nm, in good agreement with the (101) plane of Co3Sn2. The highly distributed structure of the Co3Sn2 intermetallic is proven by the uniform distribution of Co and Sn elements from energy-dispersive X-ray spectroscopy (EDS) mapping (Fig. 1f and g). It can be seen that the Co and Sn elements are uniform dispersed and coexisted over a whole polygonal Co3Sn2 nanostructure unit.
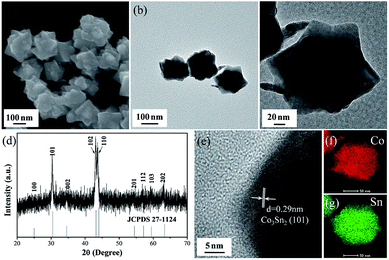 | ||
| Fig. 1 (a) SEM image, (b) TEM image, (c) HRTEM image, (d) XRD pattern, (e) HRTEM image with measured d-spacing of the selected portion of the Co3Sn2 polygon, EDS mapping of (f) Co and (g) Sn. | ||
The formation process of the polygonal Co3Sn2 nanostructure is investigated by characterizing the products acquired at different reaction times. The time-dependent morphology of the products is demonstrated in Fig. 2a–e. Before heating, the Co(OH)2 nanoplates are obtained (Fig. 2a). When the time exceeds 1 h (Fig. 2b), a heap of congregated products are gathered. Keeping under observation the surface of the congregated products, a few polygonal nanostructures begin to form. With the time going on (Fig. 2c to e), the congregated products is continuously separated to monodispersed polygons. When the times reach to 12 h, the completely monodispersed polygons are exhibited. Corresponding to the morphology evolution, the XRD patterns of the samples obtained at different duration times are displayed in Fig. 2f. It can be seen that the solid products formed before heating are Co(OH)2 and SnO. However, with the heating time increasing to 1 h, the peaks of Co(OH)2 disappear thoroughly while the peaks of SnO decrease. At the meantime, the peaks of Co and Co3Sn2 arise with strong intensity. When the time exceeds 3 h, the peaks of Co3Sn2 are obviously remarkable than that of Co and SnO. With the time increasing to 8 h and even 12 h, there are nearly the peaks of Co3Sn2 alone.
The SEM images and XRD patterns imply the formation process of the Co3Sn2 polygon. Firstly, after mixing of the solutions, the Co(OH)2 nanoplates and SnO are created. Then the Co nanoplates is in situ transformed derived from the Co(OH)2 under the solvothermal condition and the reducing action of hydrazine hydrate. Subsequently, the Sn is reduced on the surface and the corners of heaped Co nanoplates by the reducing action of hydrazine hydrate and the catalytic action of formed Co as well as the PVP-assisted active agent. Under the solvothermal condition, atomic migration is occurred between reduced Sn atoms and Co matrixes to form alloyed Co3Sn2, meanwhile, separated to form monodispersed polygons. With the continuous reaction times, the Co3Sn2 polygons are fully formed with increased crystallinity and monodispersity.
Notably, the XRD patterns (Fig. 2f) present that the Co is reduced in advance of the Sn, and then acted as a catalyst to assist the reduction of Sn from SnO. In our experimental results, only the Sn dichloride in the precursor system is hard to be reduced to metallic Sn without adding of Co dichloride. Furthermore, although the designed atomic ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co greater than 2
Co greater than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the obtained products are still Co3Sn2. The two results reveal that the pre-reduced Co in the synthetic process is instrumental to the formation of Sn and Co–Sn alloy. However, this helpfulness is not unlimited.
3, the obtained products are still Co3Sn2. The two results reveal that the pre-reduced Co in the synthetic process is instrumental to the formation of Sn and Co–Sn alloy. However, this helpfulness is not unlimited.
In addition, the PVP-assisted active agent is also a significant effect to the formation of Co3Sn2 polygon. The obtained products with different addition of PVP are shown in Fig. S1 (ESI†). Without PVP, the formation of Co3Sn2 polygon is accompanied by many small granules, while with PVP, these small granules disappear. This result suggests that the PVP absorbed on the surface of Co, as a surfactant, help the reduction of Sn onto the surface of pre-reduced Co. Moreover, as presented in Fig. S2 (ESI†), different reducing agents like NaH2PO2, propylene glycol and hydrazine hydrate all gain the Co3Sn2 alloys, but only the hydrazine hydrate gives the polygonal Co3Sn2 intermetallic. Analogously, in different solvent system like ethyl alcohol, ethylene glycol and mixed solvent of ethyl alcohol and ethylene glycol in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, only the ethylene glycol gives the polygonal Co3Sn2 intermetallic (Fig. S3, ESI†). Furthermore, no polygonal Co3Sn2 formation at 100 °C confirms that the solvothermal condition is a key factor for the formation of Co3Sn2 polygon. Notely, in this one-pot synthesis approach, the particle size of the Co3Sn2 polygon can be adjusted by changing the concentration of CoCl2·6H2O, as shown in Fig. S4 (ESI†).
1, only the ethylene glycol gives the polygonal Co3Sn2 intermetallic (Fig. S3, ESI†). Furthermore, no polygonal Co3Sn2 formation at 100 °C confirms that the solvothermal condition is a key factor for the formation of Co3Sn2 polygon. Notely, in this one-pot synthesis approach, the particle size of the Co3Sn2 polygon can be adjusted by changing the concentration of CoCl2·6H2O, as shown in Fig. S4 (ESI†).
Significantly, our experimental results present that the Sn doping has not only an influence on its structural characteristics, but also an impact on shape anisotropies of Co. As shown in Fig. 3a and b, the Co and Co3Sn2 prepared with similar solvothermal method exhibit extremely different structural characteristics. A standard FCC structure with lattice parameters refined as a = b = c = 3.545 Å and α = β = γ = 90° is possessed by the Co sample. It is obvious that the Co has the superduper tetragonality in structure. However, the Co3Sn2 has a Ni2In type hexagonal lattice in the P63/mmc space group with lattice parameters of a = b = 4.109 Å, c = 5.180 Å, α = β = 90° and γ = 120°.21–23 Distinctly, the Co3Sn2, in comparison with the Co, exhibits appreciable changes in tetragonality along the c lattice and the γ crystal plane angle. Such the changes in tetragonality gives the anisotropic crystalline structure in Co3Sn2, drastically different from the isotropous case of Co. Apart from the structural anisotropies, the shape anisotropies also occur on the Co3Sn2 polygons compared with the Co case, as revealed in Fig. 3c and d. The Co sample displays the quasi-spherical 0D morphology with the particle sizes ranging from 1 to 2 μm. But to Co3Sn2, after Sn-doping, it presents 3D polygonal morphology with reduced particle sizes of approximately 100–200 nm. Summarize that the Sn-doping endows the Co3Sn2 with structural and morphological anisotropies as well as reduced gain sizes. As to be shown below, such the anisotropic structure and morphology lead to improved magnetic properties of this material.
 | ||
| Fig. 3 XRD patterns and unit cell of (a) Co and (b) Co3Sn2; SEM images of (c) Co and (d) Co3Sn2 prepared with similar solvothermal process. | ||
The magnetic behavior of as-synthesized samples is measured using a vibrating sample magnetometer at 300 K. As shown in Fig. 4, the saturation magnetization (Ms) of the Co3Sn2 polygon is 134.01 emu g−1, higher than that of the Co sample with Ms of 74.57 emu g−1. In the low-field region, a hysteresis loop exists in both the Co3Sn2 and Co products, indicating a ferromagnetic component. As shown in the lower-right inset of Fig. 4, the coercivity (HC) of the Co3Sn2 sample is determined as 131.5 Oe (1 Oe = 103/(4π) A m−1), which is a great enhancement from the Co microsphere (HC = 31.3 Oe). Furthermore, the HC becoming as large as 131.5 Oe, it is also higher than that of other Co-, Sn-based and isostructural Ni2In-typed materials, as listed in the Table 1. By comparing the Co case, the enhancement of coercivity of Co3Sn2 sample is owing to the following factors. Firstly, the structural anisotropy from cubic FCC to hexagonal Ni2In type could be considered an essential factor; analogously, W. Han et al. find that the anisotropic FeSn5 with quasi-one dimensional crystal structure leads to different magnetic properties in comparison with the 3D case of FeSn2.19 Secondly, the increase in shape anisotropy from 0D quasi-spherical aggregates to 3D polygons may contribute to the enhanced coercivity, which is in agreement with the previous reported literature.15,17,18 Besides, the high coercivity can also be attributed to the reduced gain size of as-prepared samples from micrometer to nanometer.
 | ||
| Fig. 4 Magnetic hysteresis loop of the Co microsphere and Co3Sn2 polygon measured at 300 K; the inset shows the low field part of the hysteresis loop. | ||
| Type | Materials | Coercivity (Oe) | Reference |
|---|---|---|---|
| Co-based | Bulk Co | 10 | 24 |
| Co nanoparallelepipeds | 120 | 25 | |
| Hollow cobalt mesospheres | 66 | 26 | |
| Handkerchief-like Co48Ni52 | 31.18 | 9 | |
| Hierarchical CoNi spheres | 52.71 | 27 | |
| Flower-like Ni48Co52 spheres | 84.27 | 27 | |
| Sn-based | FeSn2 nanospheres | ∼0 | 19 |
| Fe2CoSn Heusler alloy | 48 | 28 | |
| Ni2In-type | Mn1.05Ni0.85−xCoxGe (x < 0.85) | <100 | 29 |
| MnCo1−xGe (x = 0.1 or 0.2) | <4 | 30 | |
| Co3Sn2 polygon | 131.5 | This work |
We also study the cell performance of the Co3Sn2 polygon as an anode material for lithium ion batteries. The electrochemical cycle performances of the Co3Sn2 polygon are shown in Fig. S5 (ESI†). Because of the low Sn concentration, a lower specific discharge capacity of Co3Sn2 polygon has been achieved. The first discharge and charge capacity are 758 and 360 mA h g−1, with high irreversible capacity loss in the first cycle which may be attributed to the formation of solid electrolyte interphase (SEI) film on increased surface of prepared sample. The high Co concentration endows the excellent cycling stability and rate capacities of the Co3Sn2 polygon. A reversible discharge capacity of 240 mA h g−1 is retained with high coulombic efficiency even after 200 cycles, which is higher than that of the Co sample without electrochemical lithium activity. Furthermore, even at the high current density of 1600 mA g−1, average discharge capacities of 168 mA h g−1 can still be delivered. Although the specific capacity of this material is low, it is also encouraging in consideration of its excellent cycling stability and rate capacities.
In summary, monodispersed polygonal Co3Sn2 intermetallic and Co microsphere have been successfully synthesized via a facile one-pot solvothermal route free of any template. Compared with the pure Co sample, the Co3Sn2 intermetallic exhibits structure anisotropy from cubic FCC to hexagonal Ni2In type and shape anisotropy from 0D quasi-spherical aggregates to 3D polygons. In addition, the multidimensional and anisotropic morphology and structure endow the Co3Sn2 intermetallic with enhanced magnetic properties in comparison with cubic cobalt alone. Moreover, the high Co concentration also reinvest the Co3Sn2 intermetallic with low specific capacity but good cycling life and rate capability when used as an anode material in lithium ion batteries.
Acknowledgements
This work is supported by the National Science Foundation of China (Project No. 11204102, 20111061, 21373198, and 51475207), the station Foundation of Ministry of Education for New Teachers (Project No. 20120061120041), Open Project of State Key Laboratory of Superhard Materials (Project No. 201410), and the China Postdoctoral Science Foundation (Grant No. 2015m580253).Notes and references
- V. F. Puntes, K. M. Krishnan and A. P. Alivisatos, Science, 2001, 291, 2115–2117 CrossRef CAS PubMed.
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845–910 CrossRef CAS PubMed.
- X. Wang, H. B. Fu, A. D. Peng, T. Y. Zhai, Y. Ma, F. L. Yuan and J. N. Yao, Adv. Mater., 2009, 21, 1636–1639 CrossRef CAS.
- Y. F. Lu, H. Y. Fan, C. J. Brinker, T. L. Ward and T. Rieker, Abstracts of Papers of the American Chemical Society, 1999, 218, U426 Search PubMed.
- H. L. Niu, Q. W. Chen, H. F. Zhu, Y. S. Lin and X. Zhang, J. Mater. Chem., 2003, 13, 1803–1805 RSC.
- Z. W. Liu, P. C. Chang, C. C. Chang, E. Galaktionov, G. Bergmann and J. G. Lu, Adv. Funct. Mater., 2008, 18, 1573–1578 CrossRef CAS.
- H. Z. Zhang, B. Chen and J. F. Banfield, J. Phys. Chem. C, 2010, 114, 14876–14884 CAS.
- N. A. M. Barakat, K. A. Khalil, I. H. Mahmoud, M. A. Kanjwal, F. A. Sheikh and H. Y. Kim, J. Phys. Chem. C, 2010, 114, 15589–15593 CAS.
- M. Wen, Y. F. Wang, F. Zhang and Q. S. Wu, J. Phys. Chem. C, 2009, 113, 5960–5966 CAS.
- Q. Y. Liu, X. H. Guo, Y. Li and W. J. Shen, Langmuir, 2009, 25, 6425–6430 CrossRef CAS PubMed.
- Q. Y. Liu, X. H. Guo, Y. Li and W. J. Shen, J. Phys. Chem. C, 2009, 113, 3436–3441 CAS.
- N. Mahmood, C. Z. Zhang, F. Liu, J. H. Zhu and Y. L. Hou, ACS Nano, 2013, 7, 10307–10318 CrossRef CAS PubMed.
- C. G. Yang, D. W. Zhang, Y. B. Zhao, Y. H. Lu, L. Wang and J. B. Goodenough, J. Power Sources, 2011, 196, 10673–10678 CrossRef CAS.
- X. H. Yu, A. N. Jiang, H. Y. Yang, H. S. Meng, P. Dou, D. A. Ma and X. H. Xu, Appl. Surf. Sci., 2015, 347, 624–631 CrossRef CAS.
- Y. Wang, Q. S. Zhu and H. G. Zhang, J. Mater. Chem., 2006, 16, 1212–1214 RSC.
- Y. Wang, Q. S. Zhu and L. Tao, CrystEngComm, 2011, 13, 4652–4657 RSC.
- Z. P. Liu, S. Li, Y. Yang, S. Peng, Z. K. Hu and Y. T. Qian, Adv. Mater., 2003, 15, 1946–1948 CrossRef CAS.
- X. M. Ni, Q. B. Zhao, D. G. Zhang, D. D. Yang and H. G. Zheng, J. Cryst. Growth, 2005, 280, 217–221 CrossRef CAS.
- X. L. Wang, M. Feygenson, H. Y. Chen, C. H. Lin, W. Ku, J. M. Bai, M. C. Aronson, T. A. Tyson and W. Q. Han, J. Am. Chem. Soc., 2011, 133, 11213–11219 CrossRef CAS PubMed.
- T. Scholz and R. Dronskowski, Inorg. Chem., 2015, 54, 8800–8807 CrossRef CAS PubMed.
- A. K. Larsson, R. L. Withers and L. Stenberg, J. Solid State Chem., 1996, 127, 222–230 CrossRef CAS.
- A. Leineweber, O. Oeckler and U. Zachwieja, J. Solid State Chem., 2004, 177, 936–945 CrossRef CAS.
- A. Leineweber, J. Solid State Chem., 2009, 182, 1846–1855 CrossRef CAS.
- S. Chikazumi, Physics of Magnetism, Wiley, New York, 1964 Search PubMed.
- K. M. Nam, J. H. Shim, H. Ki, S. I. Choi, G. Lee, J. K. Jang, Y. Jo, M. H. Jung, H. Song and J. T. Park, Angew. Chem., Int. Ed., 2008, 47, 9504–9508 CrossRef CAS PubMed.
- L. Guo, F. Liang, X. G. Wen, S. H. Yang, L. He, W. Z. Zheng, C. P. Chen and Q. P. Zhong, Adv. Funct. Mater., 2007, 17, 425–430 CrossRef CAS.
- M. W. Wei, X. M. Zhou, K. L. Wu and Y. Chen, CrystEngComm, 2011, 13, 1328–1332 RSC.
- S. J. Gao, Y. C. Liu and X. L. Kou, Int. J. Electrochem. Sci., 2015, 10, 8727–8737 CAS.
- X. G. Zhao, M. Tong, C. W. Shih, W. C. Chang, W. Liu and Z. D. Zhang, IEEE Trans. Magn., 2014, 50, 2504904 Search PubMed.
- Y. K. Fang, J. C. Yeh, W. C. Chang, X. M. Li and W. Li, J. Magn. Magn. Mater., 2009, 321, 3053–3056 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05077h |
| This journal is © The Royal Society of Chemistry 2016 |

