Synthesis and visible light responsed photocatalytic activity of Sn doped Bi2S3 microspheres assembled by nanosheets†
Yifan Jiang,
Juncheng Hu* and
Jinlin Li
Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission, Ministry of Education, South-Central University for Nationalities, Wuhan 430074, Hubei Province, PR China. E-mail: jchu@mail.scuec.edu.cn; Tel: +86 27 67841302
First published on 12th April 2016
Abstract
Tin-doped Bi2S3 (TDB) microspheres assembled by nanosheets with different Sn contents were synthesized via a simple one step solvothermal method. These photocatalysts were characterized by XRD, SEM, TEM, BET, XPS and DRS. The results showed that the TDBs with a portion of Sn4+ substitution at Bi3+ sites in Bi2S3 lattice were consist of nanosheets, Sn dopant can increase the specific surface area and range of light response. What's more, the possible formation mechanism of the unique structure was discussed. It exhibits that the introduction of Sn can improve the photocatalytic activity, and 3 mol% is the optimal content of doped Sn for the degradation of RhB under visible light (λ > 420 nm) irradiation. Compared to the un-doped Bi2S3, the enhanced photocatalytic activities of the TDBs might be attributed to the photo-generated electrons and holes recombination were prevented by doping Sn. The EIS measurements further confirmed the better photogenerated charge carrier separation and transport efficiency of TDBs samples. Moreover, the possible mechanism of photocatalysis process and the possible active species were investigated in detail. This photocatalyst also showed good reusability during the recycled experiments.
1. Introduction
Semiconductor-based photocatalysis, which is applied in the photocatalytic degradation of organic pollutants, has attracted enormous interest because it is inexpensive and environmentally friendly.1–4 So far, the photocatalysts based on TiO2 have been extensively studied.5 However, it has large band gap that TiO2 only works under UV light irradiation, and it has a high recombination rate of photo-generated electron–hole pairs, which results in a low photocatalytic activity.6–11 Therefore, other photocatalysts with narrow band gap should be developed to promote the usage of solar energy for photocatalytic degradation of organic pollutants under visible light.Bismuth sulfide (Bi2S3) possessing remarkable broad light absorption region because of the narrow-band-gap (1.3–1.7 eV),12–15 has many potential applications, such as X-ray-computed tomography imaging,16 gas sensors,17 electrochemical hydrogen storage,18,19 lithium ion batteries,20 visible-wavelength photodetectors21,22 and photovoltaics.23 Since the controllable morphology and the formation of structure become a significant issue in nano-materials field, considerable efforts have been devoted to synthesize different morphology and structure of Bi2S3, hierarchical disc-like Bi2S3 networks composed of perpendicularly aligned single-crystalline nanorods were produced by a novel 2D-template method,24 Bi2S3 nanowires were prepared by a modified composite molten salt technique,25 Bi2S3 nanostructures were synthesized via an anodic alumina membrane template,26 and nanotubes,27 microbelts28 of Bi2S3 have been prepared.
Recently, a lot of photocatalysts based on Bi2S3 with enhanced activity have been reported, the main factor of the increased photocatalyst activity is that the as-prepared photocatalysts can facilitate generation of the photo generated electron–hole and capture the electrons to decrease the recombination rate of electron–hole. For example, Bi2S3/g-C3N4 composite photocatalyst was produced and discussed the enhancement of RhB degradation,29 Bi2S3 nanocrystals/BiOCl heterojunctions were prepared to degrade dye,30 different 1D Bi2S3 nanostructures were synthesized to show photocatalytic reduction of Cr(VI) under visible light irradiation.31 Beyond that, many metal and nonmetal ions doping are demonstrated to improve photocatalytic activity. Se-doped Bi2S3 photocatalyst was synthesized to degrade MB under visible light.32 Eu3+ doped Bi2S3 nanoparticles were manufactured via a method of solvothermal decomposition, with the mechanism of the degradation of phenol and substituted phenols discussed.33 However, Eu is a rare-earth materials and it is expensive, other rich and economical elements should be introduced to the Bi2S3 system to enhance photocatalytic activity.
Tin (Sn) resource is abundant in China, and Sn is inexpensive and easy to get. In addition, Sn is a doubly-ionized donor, it can afford donor ion, gain higher electron carrier content and even change the band gap and the optical properties of the photocatalysts.34,35 In this work, microspheres Bi2S3 and TDBs composed of nanosheets were obtained through a simple one step solvothermal route. Furthermore, a possible formation mechanism was discussed. It is amazed to find that the doped Sn4+ can boost the activity of photocatalytic degradation of RhB, which is superior to commercial P25 and previously reported in literature.29 In addition, it is experimentally proved that 3 mol% TDB possesses a high specific surface area, wide photoabsorption range and Sn4+ dopant could effectively prevent the photogenerated electron–hole recombination. The possible active species and the reusability of the photocatalyst were investigated in detail.
2. Experimental
2.1 Chemicals and materials
Bismuth nitrate (Bi(NO3)3·5H2O), tin tetrachloride (SnCl4·5H2O), rhodamine-B (RhB) and benzyl alcohol (C6H5CH2OH) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Oleylamine and benzyl mercaptan (C7H8S) were provided by Aladdin Chemistry Co., Ltd (Shanghai, China). All chemicals were analytical reagent grade without further purification. Degussa P25 was obtained from Degussa (China) Co., Ltd. Deionized water and ethanol were used in this work.2.2 Synthesis of pure Bi2S3 and TDBs microspheres
In a typical synthesis, 6 mL oleylamine, appropriate molar ratios of Bi(NO3)3·5H2O and SnCl4·5H2O with the total mole number of Bi3+ and Sn4+ being 2 mmol were dispersed in 60 mL benzyl alcohol. The Sn-doping concentrations were designed as 0, 1.0, 2.0, 3.0, 4.0, and 5.0 mol%, which was the mole ratio of the theoretical yield. After being stirred for 1 h, the amount of the corresponding benzyl mercaptan was added to the above solution under vigorous stirring. After stirring for 0.5 h, the solution was transferred into a 100 mL Teflon-lined stainless steel autoclave, which was then heated to 180 °C and maintained for 5 h. Subsequently, the autoclave was cooled to room temperature naturally. The obtained samples were filtered, washed with deionized water and ethanol for several times, and then dried at 60 °C in air for 12 h for further characterization.2.3 Characterization methods
The structure and phase of the samples were characterized by power X-ray diffraction (XRD) employing a scanning rate of 0.05° S−1 in the 2θ range from 10° to 80°, in a Bruker D8 Advance using mono chromatic Cu Kα radiation (λ = 1.5404 Å). The microstructure and sizes of the samples were observed using an SU8000 field-emission scanning electron microscope (FESEM, Hitachi, Japan) at an accelerating voltage of 15 kV. The energy dispersive spectrum analysis (EDS) results of the catalysts were collected during the SEM measurement. A transmission electron microscope (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained by using a Tecnai G 20 microscope operated at an accelerating voltage of 200 kV. The specimens were dispersed in ethyl alcohol and dropping a drop of very dilute suspension onto a carbon film-coated copper grid. The Brunauer–Emmett–Teller (BET) specific surface area were measured on the basis of nitrogen adsorption isotherms using a Micromeritics ASAP 2020 gas adsorption apparatus (USA). X-ray photoelectron spectroscopy (XPS) was recorded on a VG Multilab 2000 (VG Inc.) photoelectron spectrometer using monochromatic Al Kα radiation under vacuum at 2 × 10−6 Pa. All the binding energies were referenced to the C 1s peak at 284.8 eV of the surface adventitious carbon. The UV-vis diffused reflectance spectra (DRS) were collected using a Shimadzu UV-2550 spectrophotometer from 300 to 800 nm using BaSO4 as a reference materials. Electrochemical impedance spectra (EIS) were performed by an electrochemical system (CHI-660e, CHI Shanghai, Inc.) in three-electrode quartz cells with 5 mmol L−1 [Fe(CN)6]3−/4− electrolyte solution, and platinum wire was used as the counter electrode, saturated calomel electrodes (SCE) were used as the reference electrodes, respectively.2.4 Photocatalytic measurements
The photocatalytic activity of the samples were tested by the degradation of RhB (50 mL, 20 mg L−1) under visible light irradiation, respectively. In each experiment, 50 mg of catalysts and RhB solution were mixed in a Pyrex glass vessel. The suspensions were magnetically stirred in the dark for 1 h to ensure the establishment of an adsorption–desorption equilibrium between the dye and the catalyst, then the mixture was vertically irradiated using a 350 W Xe lamp illumination with restricted visible-light radiation by a 420 nm cutoff filter, and the reaction cell was cooled by recycled water outside. 3 mL solution was sampled at designated irradiation time intervals, the liquid was centrifuged and then filtered through a Millipore filter (pore size 0.45 μm) to remove the catalyst particulates. All experiments are tested in the same condition. The filtrates were finally analyzed using a UV-vis spectrophotometer (UV-2550).3. Results and discussion
3.1 Crystal structure and morphology
The as-prepared samples with different Sn contents were characterized by XRD, and the typical diffraction patterns are shown in Fig. 1. All of these diffraction patterns are similar and can be easily assigned to orthorhombic Bi2S3 with lattice constants of a = 11.15 Å, b = 11.30 Å, and c = 3.98 Å (JCPDS no. 17-0320), implying the successful preparation of the Bi2S3. No diffraction peaks of tin species can be seen and no shift of the typical diffraction peaks can be measured, which suggest the low contents of Sn have little effect on the Bi2S3 lattice. | ||
| Fig. 1 XRD diffraction patterns of pure Bi2S3, 1 mol% TDB, 2 mol% TDB, 3 mol% TDB, 4 mol% TDB and 5 mol% TDB. | ||
The structure and morphology of the products were studied by SEM. Fig. 2(a, b, d and e) and Fig. S1(a–d) (ESI†) show that all the microspheres with porous structures have an average diameter of 1.5–2 μm regardless of Sn doped or not, it means that Sn has little influence on the size of spheres. It is obvious that the spheres were formed by closely packed sheets in TDBs and loosely stacked in the pure one (Fig. 2(b)), in other word, the pore volumes of spheres in TDBs are bigger than undoped one, which reveals that the doping of Sn4+ may adjust the pore volume and specific surface area. The SEM-EDS area scans (Fig. 2(c and f)) was adopted to verify the element content on the surface of the samples, it is calculated that the molar ratio of S/Bi in the undoped and 3 mol% TDB samples are 1.41 and 1.45, respectively, which are close to the Bi2S3 stoichiometry. The mass ratio of Sn in the 3 mol% TDB is 1.32%, which comes up to the rate of charge.
 | ||
| Fig. 2 SEM images of (a and b) pure Bi2S3 and (d and e) 3 mol% TDB, EDS area scans of (c) pure Bi2S3 and (f) 3 mol% TDB. | ||
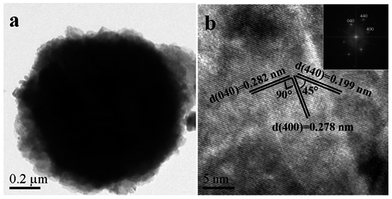
The more detailed structure of 3 mol% TDB was determined through TEM and HRTEM techniques. It is obvious that the microspheres is consist of nanosheets as revealed in the low-magnification TEM image (Fig. 3(a)), which is consistent with the SEM results. Fig. 3(b) is the HRTEM image of 3 mol% TDB sample, the clear fringe spacing with an interval of 0.282 nm, 0.278 nm and 0.199 nm can be seen obviously, which could be indexed to (040), (400) and (440) plane of orthorhombic Bi2S3 lattice according to the XRD results. The angles between (040) and (400), (400) and (440) planes are 90° and 45°, respectively, which is in agreement with the theoretical value. The angles in the corresponding fast Fourier transform (FFT) pattern (inset of Fig. 3(b)) are identical to the theoretical value, which indicates that the catalyst has a high crystallinity.
3.2 BET measurement
The isotherms of N2 adsorption–desorption of pure Bi2S3 and 3 mol% TDB microspheres were shown in Fig. 4. Both of the samples show type-IV isotherms, the hysteresis loops start at P/P0 = 0.5 and show high adsorption at P/P0 = 1.0, it reveals that the products is porous, and the porous structures may be formed by assembled Bi2S3 nanosheets. As shown in Table S1,† the specific surface areas of pure Bi2S3 and 3 mol% TDB are 10.5 and 16.6 m2 g−1, respectively. The total pore volumes of pure Bi2S3 and 3 mol% TDB are 0.045 and 0.071 cm3 g−1, respectively, the BET results are consonant with SEM images. As is well known that a higher surface area can increase the quantity of active sites and promotes the photocatalytic activity,36–38 which is agree with the subsequent photocatalytic degradation result.3.3 Possible formation mechanism of TDBs microspheres
Oleylamine is an important surfactant, it is reported that oleylamine is vitally important in formation of the morphology through regulating the counterbalance between crystal strain and surface energy.39 For comparison, we conduct a series of experiment with different amounts of oleylamine, the SEM results are shown in Fig. S2.† Obviously, the nanorods would be assembled in the absent of oleylamine, when the amount of oleylamine is less or more than 6 mol in 60 mL benzyl alcohol, the Bi2S3 could not form regular microspheres. On the basis of the literature and experimental result, the unique morphology of this sample may attribute to the corporate effects of oleylamine and benzenes (benzyl alcohol and benzyl mercaptan in this work). The possible formation mechanism of the TDB microspheres was inferred (Scheme 1): Mn+ (Bi3+ and Sn4+) were dispersed into benzyl alcohol solution, after benzyl mercaptan injected, the system became yellow, which may be Mn+–benzenes complex formed. Then the complex decomposed and turned into black Sn4+/Bi2S3 nuclei in the solvothermal process. With the help of oleylamine, the nuclei grew into a mass of nanosheets and then assembled into microspheres.3.4 Surface composition
XPS spectroscopy, which is known for its high surface sensitivity, was performed to explore the chemical composition and chemical state of the products. Fig. 5(a) clearly indicates that the catalysts are mainly composed of Bi, S elements, Sn element exists in 3 mol% TDB, and no other elements can be detected. Fig. 5(b) is the high-resolution XPS of 3 mol% TDB, the spin–orbit components (Bi 4f7/2 and Bi 4f5/2) of the peaks were well deconvoluted by two curves at approximately 159.4 and 165.0 eV, respectively, which are assigned to Bi3+. Two weak Bi 4f7/2 and Bi 4f5/2 peaks at 156.4 and 163.3 eV, respectively, which are characteristics of metal Bi, and the accurate calculation result indicates that the Bi element mainly existed as the chemical state of Bi3+, the weak peak located at 160.0 eV can be ascribed to S 2p signal, which is lower than the reported in literature.40 Two different S 2s peaks of pure Bi2S3 and 3 mol% TDB were showed in Fig. 5(c), the peak located at 225.2 eV is characteristics of S2− in pure Bi2S3, for comparison, the binding energies of S2− in 3 mol% TDB is 224.9 eV, which is lower than that of pure Bi2S3. This could be ascribed to some Bi3+ replaced by Sn4+ of the Bi2S3 lattice and the Bi–S–Sn bonds formed in the 3 mol% TDB catalyst. The XPS results further confirm that the Sn4+ substitutes Bi3+ sites in Bi2S3 lattice, which is in accord with SEM-EDS results. Fig. 5(d) provides the information of binding energies of the surface Sn element of 3 mol% TDB, the curve can be ascribed four peaks, the peaks at 495.5 and 487.1 eV corresponded to the Sn 3d3/2 and Sn 3d5/2 of Sn4+, the peaks at 493.0 and 484.7 eV can be assigned to Sn 3d3/2 and Sn 3d5/2 of metal Sn. Because of the reducibility of oleylamine,41 a spot of Bi3+ and Sn4+ could be restored to elementary substance inevitable and they attached to the surface of Bi2S3. | ||
| Fig. 5 (a) Survey XPS spectrum of the products, high-resolution XPS spectrum of (b) Bi 4f and S 2p of 3 mol% TDB, (c) S 2s of 3 mol% TDB and pure Bi2S3 and (d) Sn 3d of 3 mol% TDB. | ||
3.5 Optical properties
The optical properties of pure Bi2S3 and 3 mol% TDB were investigated by the UV-vis absorption spectroscopy and the corresponding plots are shown in Fig. S3.† Due to the black pure Bi2S3 and the TDBs products, the absorption measurements show strong photoabsorption ranging from 300 to 800 nm (inset of Fig. S3†). One can estimate the band gap of direct band gap materials by using the following formula, (αhν)2 = A (αhν − Eg), where α, hν, and A are the absorption coefficient, photoenergy, and edge–width parameter, respectively.15 By extrapolating the linear portion of the (αhν)2 versus hν curves to the energy axis at α = 0, the Eg values of the 3 mol% TDB sample is estimated to be 1.2 eV, lower than that of undoped one (1.3 eV), which in agreement with the previous literature,12,13 although it is not an obvious improvement, the doped Sn can slightly increase the range of light response.3.6 Photocatalytic degradation of RhB
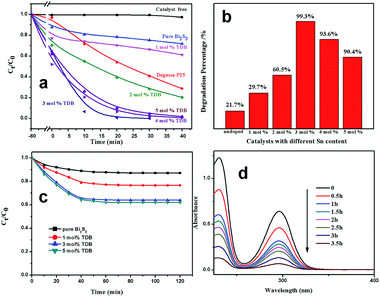
Photocatalytic degradation of RhB under visible light irradiation had been chosen as a probe reaction to evaluate the photocatalytic activities of the as-prepared samples. For comparison, commercial product Degussa P25 and blank experiment were conducted at the same experimental conditions. As can be seen from Fig. 6(a), C0 is the original concentration of RhB (20 mg L−1), Ct is the concentration of RhB after visible light irradiation for a certain period. It is known that Degussa P25 possesses low adsorptivity,38 and about 4% of RhB has been absorbed in the dark. In order to figure out the influence of adsorption, Fig. 6(c) shows the data of the experiment of dark adsorption, they reached the absorption equilibrium after about 40 min in the dark. The adsorption of as-prepared samples are different, it goes bigger with the mole ratio of Sn increased, which could owe to the enlarged specific surface area (Table S1†) by doping Sn and splendid adsorptivity of SnS2. As we know, a big specific surface area contributes to a better adsorptivity.42 In addition, the curves of degrade RhB (20 mg L−1) over pure SnS2 under visible light are shown in Fig. S4,† obviously, about 75% of RhB has been adsorbed by pure SnS2 (all the synthesis condition are the same and SnCl4·5H2O as Sn source).After adsorption–desorption equilibrium, no degradation of RhB occurred in the absence of catalyst, and there only have little degradation over pure Bi2S3 and 1 mol% TDB catalysts, whereas, the others Sn contents TDB samples show higher photocatalytic activity, especially 3 mol% TDB which is superior to commercial product Degussa P25. In order to compare the photocatalysis efficiency of each sample intuitively, the bar graph (Fig. 6(b)) was prepared. It is obviously seen that after the adsorption/desorption equilibrium in the dark, the degradation rate of RhB over pure Bi2S3, 1 mol%, 2 mol%, 3 mol%, 4 mol%, 5 mol% TDB are 21.7%, 29.7%, 60.5%, 99.3%, 93.6%, 90.4% in 20 minutes, respectively.
To investigate the photosensitization in the degradation process, compared the data of BQ in Fig. 8 with 3 mol% TDB in Fig. 6(a), after absorption equilibrium, it is about 13% of original RhB have been degraded through self-photosensitization. 3 mol% TDB sample with band gap of 1.2 eV is easily excited under visible light. To avoid photosensitization effect of degradation process, salicylic acid (20 mg L−1) was chosen as target pollutant, it also shows splendid photocatalytic activity (Fig. 6(d)), the degradation rate is about 96.4% in 3.5 h. It is evident that the doped Sn can enhance the activities of the photocatalysts. The efficient photocatalytic activity of 3 mol% doped catalyst could be mainly attributed to the incorporated Sn4+ working as electrons-captor temporary to prevent the photo-generated electrons and holes recombination and the bigger specific surface area providing more active sites on the surface. Due to Bi2S3 lattice are not greatly incorporated by Sn4+ with relatively high or low content, the photocatalytic efficiencies are not as good as 3 mol% TDB.
3.7 Possible mechanism of photocatalysis process
Generally, the band edge position could be theoretically calculated by Mulliken electronegativity definition as seen in eqn (1) and (2):43| EVB = X − Ee + 1/2Eg | (1) |
| ECB = EVB − Eg | (2) |
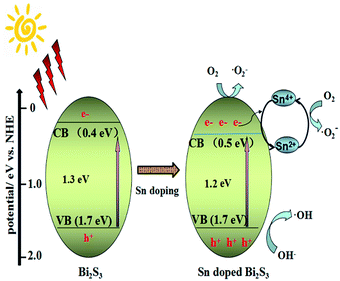
On the basis of the and experimental results and literature,45,46 the plausible mechanism of photocatalysis process is proposed (Scheme 2), when the 3 mol% TDB catalyst is irradiated under visible light, it can easily absorb visible light and be excited to produce photo-generated electron–hole pairs, and the photo-induced electrons are excited from valence band to conduction band, and captured by molecular oxygen to produce reactive oxygen radicals (˙O2−) on the Bi2S3 surface which can oxidize RhB, since Sn4+ partly entered the Bi2S3 lattice, the Sn4+ which is in priority to Bi0 and Sn0 to act as trapping site of photo-generated electrons, then it turns into Sn2+, the Sn2+ is unsteady and can be oxidized to Sn4+ by O2 and form ˙O2−,46 which can degrade RhB further. The photo-generated holes in VB of Bi2S3 can directly oxidize RhB or formed ˙OH by oxidizing OH− to oxidize RhB. To sum up, the doped Sn can promote the effective separation of photo-excited electron–hole pairs and suppress the recombination of electron–hole. Hence, the photocatalytic activity is enhanced by the doped Sn.
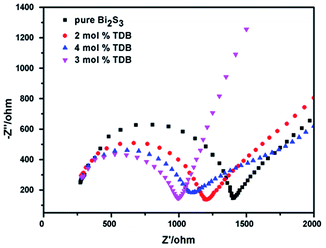
Moreover, electrochemical impedance spectra (EIS) was employed to further confirm the as-prepared catalysts whether can enhance the separation of photo-generated electron–hole and transfer efficiency.47,48 The arc radius of the Nyquist plots reflect the proposed photocatalytic mechanism (Scheme 2) of 3 mol% TDB under visible-light irradiation. The electrical resistance of photocatalysts, which reveals the recombination of electron–hole on the surface of the product. In Fig. 7, the arc radius of 3 mol% TDB is smaller than others, this imply that the sample has a relatively lower electrical resistance and effectively prevent the recombination of electron–hole, thus, the photocatalytic performance is improved. This is in good agreement with the result of previous photocatalytic experiment.
3.8 Possible active species
As we know, electrons (e−), holes (h+), hydroxyl radicals (˙OH) and superoxide radicals (˙O2−) are the possible active species in the photodegradation of organic pollutants.45 In order to detect the active species during this photocatalytic reaction, silver nitrate (AgNO3), edetate disodium (EDTA-2Na), isopropanol (IPA) and benzoquinone (BQ) were added into the RhB solution as scavengers, respectively.49,50 Fig. 8 depicts that the addition of EDTA-2Na and IPA have tiny effects in photodegradation activity of 3 mol% TDB. This demonstrates that holes and ˙OH are not the main active species in the photocatalysis process. The photodegradation efficiency is decreased when AgNO3 was added, confirming that electrons were certified as a active specie. The photocatalytic performance was significantly suppressed after BQ is introduced, indicating that ˙O2− play a vital important role in the photodegradation process.3.9 Reusability
The reusability of 3 mol% TDB is tested by RhB degradation under visible light (λ > 420 nm) for four recycles, the degradation results are shown in Fig. 9. With the repeated experiment, the degradation rate is 100%, 98.48%, 97.22%, 96% in 30 minutes, respectively, which indicates that the photocatalytic performance of the product keeps a high-level after four recycles. And the relevant XRD patterns (Fig. S5†) reveal that no distinct peaks change after the photocatalytic recycles. It indicates that the TDB photocatalyst has a good stability and reusability.4. Conclusions
In summary, microspheres Bi2S3 and TDBs composed of nanosheets with different Sn content were fabricated through a simple one step solvothermal process using benzyl alcohol as solvent, oleylamine as surfactant and in the presence of benzyl mercaptan. Possible formation mechanism of the unique morphology has been proposed. The photocatalytic activity was improved by doping Sn, and 3 mol% TDB showed the best photocatalytic efficiency. The key factors of enhanced photocatalytic activity may be as follows: (1) the Sn4+ incorporated to Bi2S3 lattice can increase specific surface area and active sites, (2) the Sn4+ dopant can facilitate the generation of photo-generated electrons–holes and work as electrons-trapper temporary to reduce their recombination rate and thus enhance the photocatalytic activity. The superoxide radicals (˙O2−) is the main active species and the catalyst showed good reusability.Acknowledgements
This work was supported by Natural Science Foundation of Hubei Province (2013CFA089).Notes and references
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed
.
- G. H. Jiang, X. Li, Z. Wei, T. T. Jiang, X. X. Du and W. X. Chen, Powder Technol., 2014, 260, 84–89 CrossRef CAS
.
- J. G. Yu, Y. R. Su and B. Cheng, Adv. Funct. Mater., 2007, 17, 1984–1990 CrossRef CAS
.
- G. H. Jiang, Z. Wei, H. Chen, X. X. Du, L. Lei, Y. K. Liu, Q. Huang and W. X. Chen, RSC Adv., 2015, 5, 30433–30437 RSC
.
- R. S. Yuan, T. Chen, E. H. Fei, J. L. Lin, Z. X. Ding, J. L. Long, Z. Z. Zhang, X. Z. Fu, P. Liu, L. Wu and X. X. Wang, ACS Catal., 2011, 1, 200–206 CrossRef CAS
.
- H. Yu, H. Irie and K. Hashimoto, J. Am. Chem. Soc., 2010, 132, 6898–6899 CrossRef CAS PubMed
.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed
.
- S. Kitano, N. Murakami, T. Ohno, Y. Mitani, Y. Nosaka, H. Asakura, K. Teramura, T. Tanaka, H. Tada, K. Hashimoto and H. Kominami, J. Phys. Chem. C, 2013, 117, 11008–11016 CAS
.
- Q. Jin, T. Ikeda, M. Fujishima and H. Tada, Chem. Commun., 2011, 47, 8814–8816 RSC
.
- T. Ohno, M. Akiyoshi, T. Umebayashi, K. Asai, T. Mitsui and M. Matsumura, Appl. Catal., A, 2004, 265, 115–121 CrossRef CAS
.
- G. H. Jiang, X. H. Wang, Z. Wei, X. Li, X. G. Xi, R. B. Hu, B. L. Tang, R. J. Wang, S. Wang, T. Wang and W. X. Chen, J. Mater. Chem. A, 2013, 1, 2406–2410 CAS
.
- G. Konstantatos, L. Levina, J. Tang and E. H. Sargent, Nano Lett., 2008, 8, 4002–4006 CrossRef CAS PubMed
.
- H. C. Liao, M. C. Wu, M. H. Jao, C. M. Chuang, Y. F. Chen and W. F. Su, CrystEngComm, 2012, 14, 3645–3652 RSC
.
- Z. Liu, S. Peng, Q. Xie, Z. Hu, Y. Yang, S. Zhang and Y. Qian, Adv. Mater., 2003, 15, 936–940 CrossRef CAS
.
- G. H. Chen, Y. Q. Yu, K. Zheng, T. Ding, W. L. Wang, Y. Jiang and Q. Yang, Small, 2015, 11, 2848–2855 CrossRef CAS PubMed
.
- O. Rabin, J. M. Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, Nat. Mater., 2006, 5, 118–122 CrossRef CAS PubMed
.
- K. Yao, W. W. Gong, Y. F. Hu, X. L. Liang, Q. Chen and L. M. Peng, J. Phys. Chem. C, 2008, 112, 8721–8724 CAS
.
- B. Zhang, X. C. Ye, W. Y. Hou, Y. Zhao and Y. Xie, J. Phys. Chem. B, 2006, 110, 8978–8985 CrossRef CAS PubMed
.
- L. S. Li, N. J. Sun, Y. Y. Huang, Y. Qin, N. N. Zhao, J. N. Gao, M. X. Li, H. H. Zhou and L. M. Qi, Adv. Funct. Mater., 2008, 18, 1194–1201 CrossRef CAS
.
- J. M. Ma, Z. F. Liu, J. B. Lian, X. C. Duan, T. Kim, P. Peng, X. D. Liu, Q. Chen, G. Yao and W. J. Zheng, CrystEngComm, 2011, 13, 3072–3079 RSC
.
- X. L. Yu and C. B. Cao, Cryst. Growth Des., 2008, 8, 3951–3955 CAS
.
- G. Konstantatos, L. Levina, J. Tang and E. H. Sargent, Nano Lett., 2008, 8, 4002–4006 CrossRef CAS PubMed
.
- R. Suarez, P. K. Nair and P. V. Kamat, Langmuir, 1998, 14, 3236–3241 CrossRef CAS
.
- L. S. Li, N. J. Sun, Y. Y. Huang, Y. Qin, N. N. Zhao, J. N. Gao, M. X. Li, H. H. Zhou and L. M. Qi, Adv. Funct. Mater., 2008, 18, 1194–1201 CrossRef CAS
.
- Q. Yang, C. G. Hu, S. X. Wang, Y. Xi and K. Y. Zhang, J. Phys. Chem. C, 2013, 117, 5515 CAS
.
- N. Petkov, J. Xu, M. A. Morris and J. D. Holmes, J. Phys. Chem. C, 2008, 112, 7345–7355 CAS
.
- J. R. Ota and S. K. Stivastava, Nanotechnology, 2005, 16, 2415–2419 CrossRef CAS PubMed
.
- Y. Zhao, X. Zhu, Y. Y. Huang, S. X. Wang, J. L. Yang and Y. Xie, J. Phys. Chem. C, 2007, 111, 12145–12148 CAS
.
- X. S. Rong, F. X. Qiu, J. Yan, H. Zhao, Z. L. Zhu and D. Y. Yang, RSC Adv., 2015, 5, 24944–24952 RSC
.
- H. Cheng, B. Huang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 97–99 RSC
.
- E. L. Hu, X. H. Gao, A. Etogo, Y. L. Xie, Y. J. Zhong and Y. Hua, J. Alloys Compd., 2014, 611, 335–340 CrossRef CAS
.
- L. M. Song, C. Chen and S. J. Zhang, Powder Technol., 2011, 207, 170–174 CrossRef CAS
.
- A. Sarkar, A. B. Ghosh, N. Saha, A. K. Dutta, D. N. Srivastava, P. Paul and B. Adhikary, Catal. Sci. Technol., 2015, 5, 4055–4063 CAS
.
- Y. Zhao, J. Liu, L. Y. Shi, S. Yuan, J. H. Fang, Z. Y. Wang and M. H. Zhang, Appl. Catal., B, 2011, 103, 436–443 CrossRef CAS
.
- W. H. Leng, Z. Zhang, J. Q. Zhang and C. N. Cao, J. Phys. Chem. B, 2005, 109, 15008–15023 CrossRef CAS PubMed
.
- W. W. Wang, Y. J. Zhu and L. X. Yang, Adv. Funct. Mater., 2007, 17, 59–64 CrossRef CAS
.
- A. Fumiaki, Y. Akira, N. Kohei, O. Masatoshim and O. Bunsho, J. Am. Chem. Soc., 2008, 130, 17650–17651 CrossRef PubMed
.
- R. J. Wang, G. H. Jiang, X. H. Wang, R. B. Hu, X. G. Xi, S. Y. Bao, Y. Zhou, T. Tong, S. Wang, T. Wang and W. X. Chen, Powder Technol., 2012, 228, 258–263 CrossRef CAS
.
- Z. Q. Niu, Q. Peng, M. Gong, H. P. Rong and Y. D. Li, Angew. Chem., Int. Ed., 2011, 123, 6439–6443 CrossRef
.
- Y. H. Shi, Y. J. Chen, G. H. Tian, H. G. Fu, K. Pan, J. Zhou and H. J. Yan, Dalton Trans., 2014, 43, 12396–12404 RSC
.
- S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS PubMed
.
- B. R. Muller, Carbon, 2010, 48, 3607–3615 CrossRef
.
- X. Y. Xiong, L. Y. Ding, Q. Q. Wian, Y. X. Li, Q. Q. Jiang and J. C. Hu, Appl. Catal., B, 2016, 188, 283–291 CrossRef CAS
.
- H. Chen, L. Y. Ding, W. Sun, Q. Q. Jiang, J. C. Hu and J. L. Li, RSC Adv., 2015, 5, 56401–56409 RSC
.
- G. H. Jiang, R. J. Wang, X. H. Wang, X. G. Xi, R. B. Hu, Y. Zhou, S. Wang, T. Wang and W. X. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4440–4444 CAS
.
- F. X. Xie, X. M. Mao, C. M. Fan and Y. W. Wang, Mater. Sci. Semicond. Process., 2014, 27, 380–389 CrossRef CAS
.
- X. J. Bai, L. Wang and Y. F. Zhu, ACS Catal., 2012, 2, 2769–2778 CrossRef CAS
.
- X. Xiao, R. Hu, C. Liu, C. Xing, C. Qiao, X. Zuo, J. Nan and L. Wang, Appl. Catal., B, 2013, 140–141, 433–443 CrossRef CAS
.
- L. Mohapatra, K. Parida and M. Satpathy, J. Phys. Chem. C, 2012, 116, 13063–13070 CAS
.
- Z. Wei, G. H. Jiang, L. Shen, X. Li, X. H. Wang and W. X. Chen, MRS Commun., 2013, 3, 145–149 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of TDBs, photocatalytic degradation with different scavengers and XRD pattern of fresh and used photocatalysts. See DOI: 10.1039/c6ra02621d |
| This journal is © The Royal Society of Chemistry 2016 |