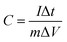RuO2@Co3O4 heterogeneous nanofibers: a high-performance electrode material for supercapacitors†
Decai Gong,
Jian Zhu and
Bingan Lu*
School of Physics and Electronics, Hunan University, Changsha, 410082, P. R. China. E-mail: luba2012@hnu.edu.cn
First published on 4th May 2016
Abstract
Novel RuO2@Co3O4 heterogeneous nanofibers (HNFs) were synthesized by a simple electrospinning method, followed by calcination. The possible mechanism of formation of RuO2@Co3O4 HNFs is presented. The synergy between RuO2 and Co3O4 and the unique feature of heterostructure allow the composite to exhibit a high reversible capacity of 1103.6 F g−1 at a current density of 10 A g−1 and a high-rate capability of 500.0 F g−1 at a current density of 100 A g−1. Moreover, excellent cycling performance was observed, where the capacities could be maintained at 93.0%, 91.8%, and 88.0% after 1000, 2000 and 5000 cycles, respectively, at a current density of 10 A g−1. This study therefore confirms that the as-prepared RuO2@Co3O4 HNFs can serve as advanced supercapacitor (SCs) materials. It is highly anticipated that this simple method of electrospinning can be extended to preparing other new types of heterostructural materials that could be used in the field of electrochemical energy storage.
1. Introduction
The demand for rechargeable battery systems is increasing, due to the growing requirement for electrical grid storage to convert the intermittent renewable sources (such as solar and wind) into electricity.1,2 Supercapacitors (SCs), a new class of energy storage devices, have attracted much attention for their high power density, fast charging/discharging rate, long cycle life and high reliability.3–7 However, the energy density of SCs is much lower than that of other batteries (e.g. Li-ion batteries).8,9 The energy density (E) of SCs depends on the cell potential (V) and capacitance (C); E = 2−1CV2.10–14 As we know, the parameters (V and C) are dominated mainly by electrode properties.15 To date, many metal oxides, such as RuO2,16 MnO2,17,18 Co3O4 (ref. 19 and 20) and NiO21 have been studied as the most promising candidates for electrode materials in the SCs because they can provide higher energy densities. Among the metal oxides, RuO2 is of significant interest as an electrode material, due to its high energy storage capabilities with large specific capacitance.22,23 However, RuO2 is not available in abundance, and this coupled with its prohibitive price, inhibit its commercial applications in SCs.24–26To solve this problem, we would need to lower the cost of the RuO2 electrode. In recent years, a number of promising attempts utilizing the combination of two kinds of metal oxides as electrode materials in SCs have been developed. Pang et al.27 described the fabrication of the Mn3O4@Co3O4 nanocubic composite for SCs with a specific capacitance of 443 F g−1 at 1.5 A g−1. Youn et al.28 reported RuO2@Mn3O4 composite nanofiber mats for SCs with a specific capacitance of 293 F g−1 at 10 mV s−1. Li et al.29 demonstrated the preparation of RuO2/Co3O4 thin films for SCs with a specific capacitance of 690 ± 14 F g−1 at 0.2 A g−1. Liu et al.30 reported Co3O4/RuO2·xH2O composites for electrochemical capacitors with a specific capacitance of 642 F g−1. Apparently, the combination of RuO2 with the appropriate metal oxide as an electrode material lowers the cost of the electrode. Besides, compared with single-phase oxides, multiple composite oxides have the advantages of high surface area and fast electron/electrolyte diffusion paths for charge storage and delivery.31,32 Among the various metal oxides, because of the outstanding electrochemical behavior, good corrosion stability and abundance, Co3O4 is considered as a promising electrode material in SCs.33–35 RuO2@Co3O4 electrodes prepared by the spray pyrolysis method and the thermal decomposition method were studied in alkaline solutions and showed good electrochemical behavior.29,30
Electrospinning is known to be a simple and facile technique, and one-dimensional (1D) nanostructures, such as nanofibers (NFs) and nanotubes (NTs) with controllable diameters and morphologies can be easily fabricated.36,37 The electrospun nano materials have a lot of unique features, such as high surface area, extraordinary length, and porous structure, which are important determining factors of the performance of SCs.38,39 Thus, we designed and rationally synthesized RuO2@Co3O4 heterogeneous nanofibers (HNFs), based on the electrospinning method, and expected to provide a promising candidate for electrode materials in the SCs.
In this paper, we report a simple electrospinning strategy to prepare the novel RuO2@Co3O4 HNFs and investigate their electrochemical properties as electrode materials for SCs. Additionally, a possible formation mechanism of RuO2@Co3O4 HNFs is proposed, based on a series of temperature-dependent experiments during the process of calcination (350, 450 and 500 °C). Compared with previous RuO2@Co3O4 composites for SCs, our prepared RuO2@Co3O4 HNFs exhibit a remarkable cycling ability of 970.5 F g−1 after 5000 cycles at the current density of 10 A g−1 (88.0% of the initial capacitance). Even when the current density reached 100 A g−1, the reversible capacity of the RuO2@Co3O4 HNFs can still maintain 500.0 F g−1. Because of the synergistic effect between RuO2 and Co3O4, as well as the unique feature of heterostructure, the electrochemical performance of the electrode has been greatly enhanced.
2. Experimental section
All the chemicals and solvents were of analytical grade and were used without further purification. Ruthenium chloride hydrate (RuCl3·3H2O) was obtained from Beijing HWRK Chemical Company Limited, and cobaltous nitrate (Co(NO3)2·6H2O) was obtained from Tianjin Chemical Corp., China. Polyvinylpyrrolidone (PVP; Mw ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich.
000) was purchased from Sigma-Aldrich.
2.1. Preparation of 1D RuO2@Co3O4 HNFs by electrospinning
In a typical synthesis, 3.3 g absolute ethanol and 3.3 g N,N-dimethyl formamide were mixed as solvent. Then, 0.104 g ruthenium chloride hydrate (RuCl3·3H2O) and 0.291 g cobaltous nitrate (Co(NO3)2·6H2O) were dissolved in the as-prepared solvent by magnetic stirring for 1 h at room temperature. The atomic ratio (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co) was about 1
Co) was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5. After complete dissolution of RuCl3·3H2O and Co(NO3)2·6H2O, 0.6 g polyvinyl pyrrolidone (PVP; Mw ≈ 1
2.5. After complete dissolution of RuCl3·3H2O and Co(NO3)2·6H2O, 0.6 g polyvinyl pyrrolidone (PVP; Mw ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was added to the resulting solution and stirred for 3 h at room temperature. The obtained solution was then loaded into a glass syringe with a pinhead. The distance between the pinhead and the collector was 18 cm and the applied voltage was 20 kV. The as-electrospun RuCl3@Co(NO3)2@PVP composite NFs were placed in a vacuum oven for 24 h at 70 °C. The spun fibers were then annealed at 500 °C for 2 h with a heating rate of 3 °C min−1 in air.
000) was added to the resulting solution and stirred for 3 h at room temperature. The obtained solution was then loaded into a glass syringe with a pinhead. The distance between the pinhead and the collector was 18 cm and the applied voltage was 20 kV. The as-electrospun RuCl3@Co(NO3)2@PVP composite NFs were placed in a vacuum oven for 24 h at 70 °C. The spun fibers were then annealed at 500 °C for 2 h with a heating rate of 3 °C min−1 in air.
2.2. Synthesis of the RuO2@Co3O4 HNFs electrode
The RuO2@Co3O4 HNFs electrode was prepared by the following process. 80 wt% of RuO2@Co3O4 HNFs, 10 wt% of carbon black and 10 wt% of polytetrafluoroethylene (PTFE, Aldrich) were mixed together. We used the Ni foam as an electrode and pressed the slurry onto it; finally, the RuO2@Co3O4 HNFs electrode was dried at 70 °C in a vacuum oven for 24 h.2.3. Characterization
The morphologies and the energy dispersive spectroscopy (EDS) mapping of the obtained samples were characterized by a field-emission scanning electron microscope (FE-SEM, Hitachi S-4800) equipped with an X-ray energy dispersive spectrometer. The crystal structures of the samples were characterized by X-ray diffraction (XRD, Philips, X'pert pro, Cu Ka, 0.154056 nm). Transmission electron microscope (TEM) and high-resolution TEM (HRTEM) investigations were carried out by a JEOL JEM-2100F microscope. In addition, X-ray photoelectric spectroscopy (XPS, ESCALAB 220i-XL, VG Scientific) was employed to analyze the surface chemical structures of the RuO2@Co3O4 HNFs.2.4. Electrochemical measurements
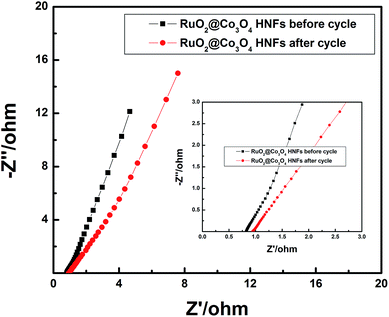
The electrochemical measurements were carried out in a three electrode electrochemical workstation with 2 M KOH aqueous solution as the electrolyte. A standard calomel electrode was used as the reference electrode and a Pt foil as the counter electrode. The RuO2@Co3O4 HNFs electrode was used as the work electrode. The mass of the active materials in the electrode was about 1 mg. The cyclic voltammetry (CV) measurements, galvanostatic charge/discharge tests and electrochemical impedance spectroscopy (EIS) were carried out using the CHI660D electrochemical workstation (Chenghua, Shanghai). Electrochemical impedance spectroscopy (EIS) measurements were performed at a frequency range of 0.01 Hz to 100 kHz. The specific capacitance (C) was calculated according to the following formula:where I is the discharge current, Δt is the discharge time, ΔV refers to the discharge range, and m represents the mass of the active electrode materials within the electrode.
3. Results and discussion
Fig. 1a shows the typical FE-SEM image of the as-spun RuCl3@Co(NO3)2@PVP NFs before annealing, which have a regular diameter size in the range of 80–160 nm. Fig. 1b shows the FE-SEM image of the as-obtained RuO2@Co3O4 HNFs after thermal treatment at 500 °C for 2 h, with a heating rate of 3 °C min−1 in air. It is obvious that the diameter of the nanofibers decreased and lots of particles were formed on the NFs. The average diameter of the NFs after annealing was 50 nm. Fig. S1† shows the FE-SEM image and corresponding EDS mapping of the RuO2@Co3O4 HNFs, revealing that mainly Ru element was distributed in the particles, and Co element was mainly distributed in the nanofibers; O element was distributed throughout the entire area. According to the EDS mapping, we confirmed that most of the RuO2 crystals formed the particles and loaded on the surface of the nanofibers (mainly consisting of Co3O4). For comparison, Fig. S2† shows the FE-SEM image of pure Co3O4 NFs, which are continuous and have a regular diameter size in the range of 70–100 nm. To further study the morphology and structure of the as-prepared RuO2@Co3O4 HNFs, the samples were examined by TEM and XRD. Fig. 1c is the TEM image of an individual RuO2@Co3O4 heterogeneous nanofiber (HNF). As shown in Fig. 1c the particle is deposited on the fiber, which agrees well with the FE-SEM observation. Insets in Fig. 1c show the HRTEM images of the RuO2@Co3O4 HNFs, which provide deep insight into the structural features of the as-prepared products. Fig. 1d is a HRTEM image of the particles on the fiber. The lattice fringes show the structural characteristics of the RuO2 crystal, in which the d-spacing of 0.32 nm corresponds to the distance of the (110) plane. Fig. 1e and f are HRTEM images of the fiber. The lattice fringes with interplane spacing of 0.47 and 0.32 nm correspond to the (111) plane of Co3O4 and (110) plane of RuO2, respectively. Fig. 1g shows the XRD patterns of the as-prepared RuO2@Co3O4 HNFs. The diffraction lines are in good agreement with Co3O4 (JCPDS No. 43-1003) and RuO2 phases (JCPDS No. 43-1027), indicating that the crystalline structures of Co3O4 and RuO2 were formed after annealing. The diffraction values at 2 theta of 19.00, 31.27, 36.85, 38.55, 44.81, 55.66, 59.35, 65.23 and 77.33° correspond to (111), (220), (311), (222), (400), (422), (511), (440) and (533) crystal plane reflections of Co3O4, respectively. The diffraction values at 2 theta of 28.02, 35.07, 40.04, 40.55, 54.27, 57.92, 59.45, 65.56, 66.98, 69.54 and 74.10° are in good agreement with (110), (101), (200), (111), (211), (220), (002), (310), (112), (301) and (202) crystal plane reflections of RuO2, respectively. The XRD patterns of pure Co3O4 NFs are shown in Fig. S3† and all the peaks were readily indexed to the Co3O4 (JCPDS No. 43-1003). Fig. S4a† is the XPS general spectra of the RuO2@Co3O4 HNFs. As shown in Fig. S4b,† there are two XPS signals of Ru 3p at binding energies around 462.5 eV (Ru 3p3/2) and 485.2 eV (Ru 3p1/2), indicating the Ru4+ bonding in RuO2.40 The peaks at 780.2 and 795.6 eV, correspond to the Co 2p3/2 and Co 2p1/2 spin–orbit peaks of Co3O4 (Fig. S4c†).41 In addition, there are two peaks can be fitted to the O 1s (Fig. S4d†), attributed to the Co–O and Ru–O bonding, respectively.In order to further understand the formation mechanism of the RuO2@Co3O4 HNFs (Fig. 2e), a series of temperature-dependent experiments during the process of calcination (350, 450 and 500 °C) was conducted and observed by FE-SEM (Fig. 2a–d). Fig. 2a shows the FE-SEM image of the as spun fibers before annealing, revealing their morphology of ordinary NFs. When the temperature increased to 350 °C, the diameter of the NFs was decreased and particle-like structures were formed on each nanofiber (Fig. 2b). This was probably due to the decomposition of PVP and crystallization of RuO2 (oxidation of RuCl3), deposited on the NFs; the NFs mainly consisted of Co3O4 (oxidation of Co(NO3)2).42 Fig. S5† shows the XRD of the spun fibers calcined at 350 and 450 °C, respectively, indicating the formation of RuO2 and Co3O4. As the temperature continued to increase, the diameter of the NFs got smaller and the particle sizes got bigger (Fig. 2c and d). This was attributed to the coalescence of the small crystal grains of RuO2. After the final calcination at 500 °C for 2 h, most of the crystal grains of RuO2 deposited on the NFs, which were composed of Co3O4 and the rest of RuO2, confirmed by EDS mapping and HRTEM images (Fig. S1† and 1d–f). To probe how the concentration of the precursor solution affected the morphology of the RuO2@Co3O4 HNFs, we prepared the RuO2@Co3O4 HNFs under the same conditions, except the high atomic ratio of Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co in the precursor solution (about 1
Co in the precursor solution (about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fig. S6† gives the FE-SEM image of the RuO2@Co3O4 HNFs (high atomic ratio of Ru
1). Fig. S6† gives the FE-SEM image of the RuO2@Co3O4 HNFs (high atomic ratio of Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co), which shows larger particles, compared to the RuO2@Co3O4 HNFs of low atomic ratio of Ru
Co), which shows larger particles, compared to the RuO2@Co3O4 HNFs of low atomic ratio of Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co.
Co.
To investigate the electrochemical capacitive performance of the RuO2@Co3O4 HNFs, a series of electrochemical measurements were carried out in a three-electrode cell with 2 M KOH as the electrolyte. The electrode using Co3O4 nanofibers was used for comparison. Fig. 3a shows the cyclic voltammograms (CVs) of the RuO2@Co3O4 HNFs from 0 to 0.6 V (vs. SCE) at different scan rates of 5, 10, 20, 40, 60, 80 and 100 mV s−1. The shapes of the CV curves are considerably different from an ideal rectangular shape. Besides, the distinct oxidation peak at about 0.52 V and the reduction peak at 0.38 V are observed when the scan rate is 5 mV s−1, indicating that the capacitance characteristics are mainly governed by pseudocapacitance, which is caused by the faradaic redox reactions of the electroactive material.43,44 With the increase of the scan rate, the anodic peaks shifted towards positive potential and the cathodic peaks shifted towards negative potential which was caused by the polarization of the electrode under high scan rates.45,46
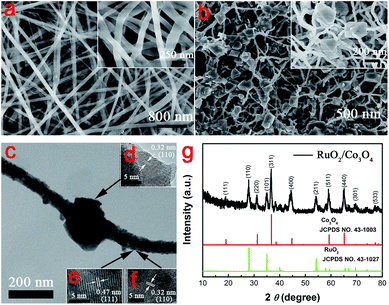
Fig. 3b displays the galvanostatic charge and discharge curves of the RuO2@Co3O4 HNFs between 0.2 and 0.55 V in 2 M KOH solution at current densities ranging from 5 to 100 A g−1. Fig. 3c displays the galvanostatic charge and discharge curves of the Co3O4 NFs. The specific capacitances of the RuO2@Co3O4 HNFs calculated according to the equation above are 1375.0, 1167.9, 1007.1, 757.1, 664.3, 571.4, 500.0 F g−1 at the current densities of 5, 10, 20, 40, 60, 80, 100 A g−1, respectively, as presented in Fig. 3d. However, the specific capacitances of Co3O4 nanofibers are 788.2, 636.2, 540.4, 454.5, 392.6, 340.1, 312.2 F g−1 at the current densities of 5, 10, 20, 40, 60, 80, 100 A g−1, respectively (shown in Fig. 3d).
The cycling stability of the RuO2@Co3O4 HNFs and pure Co3O4 electrodes were investigated at current density of 10 A g−1. As shown in Fig. 4a, the specific capacitance was about 1103.6 F g−1 in the beginning and it decreased to 970.6 F g−1, with 88.0% retention after 5000 cycles. In addition, the FE-SEM image of the RuO2@Co3O4 HNFs after the cycling test (Fig. S7†) shows that there was little change in morphology before and after the cycling test. Fig. 4b shows the high rate capability of the RuO2@Co3O4 HNFs. The specific capacitances of the electrodes were calculated for 500 cycles at the current densities of 10, 20, 40, 60, 80 and 100 A g−1, as 1078.6, 978.6, 728.6, 664.3, 571.4 and 500.0 F g−1, respectively. A recovered specific capacitance of 1021.4 F g−1 (94.7% of the initial capacitance) was calculated for the following 500 cycles, without a noticeable decrease when the current density decreased to 10 A g−1. On the other hand, the specific capacitance of the Co3O4 nanofibers were only maintained at 636.2, 540.0, 443.2, 392.5, 340.1 and 312.5 F g−1 at the current densities of 10, 20, 40, 60, 80 and 100 A g−1, respectively (Fig. 4b). The results imply that the combination of RuO2 and Co3O4 obtained by electrospinning greatly enhanced the electrochemical properties of SCs.
The improved performance of RuO2@Co3O4 HNFs electrodes can be attributed to the following aspects. First, the unique feature of the heterostructure of the RuO2@Co3O4 HNFs leads to a high specific surface area, which increases the electrolyte/electrode contact areas and reaction surface area. Thus, the electrodes can generate more active sites for fast faradaic redox reactions. Secondly, the enhanced capacity of the RuO2@Co3O4 HNFs compared with the bare Co3O4 nanofibers can be interpretated by the addition of a new capacitive component of RuO2 and the synergistic effect between RuO2 and Co3O4. The as-formed Co3O4 NFs provide a scaffold for the growth of RuO2 particles, which can avoid conventional aggregation and ensure sufficient ion diffusion. On the other hand, the deposited RuO2 possesses high specific capacitance, leading to the high specific capacitance of the RuO2@Co3O4 composite material.
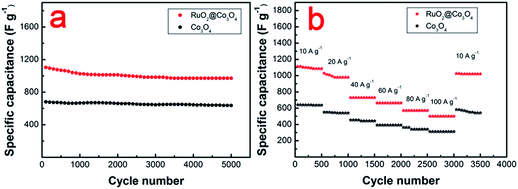
Fig. 5 shows the electrochemical impedance spectra (EIS) for the first and the 5000th cycle of the RuO2@Co3O4 HNFs electrode. At the high frequency region, both of the curves display a negligible semicircle, which indicates the charge transfer resistance is low. Apparently, there are two differences between the spectra. Firstly, in the high frequency area, the RuO2@Co3O4 HNFs electrode has larger internal resistances after 5000 cycles, revealing that the interface charge transfer of the RuO2@Co3O4 HNFs electrode shows a slight tendency to decline. Secondly, at lower frequencies, the diffusive resistance of the RuO2@Co3O4 HNFs electrode after 5000 cycles is a little higher, which indicates that the electrolyte penetration and ion diffusion in the host materials have not reduced notably.
 | ||
| Fig. 5 Electrochemical impedance spectra for the first and the 5000th cycle of the RuO2@Co3O4 HNFs electrode. Inset is the expanded view. | ||
4. Conclusion
In summary, a facile strategy has been developed to prepare RuO2@Co3O4 HNFs with high electrochemical performance for SCs. The as-prepared RuO2@Co3O4 HNFs electrode shows a high specific capacitance of 1103.6 F g−1 at 10 A g−1, with good cycling performance (88.0% of the capacity retention after 5000 cycles) and desirable rate performance (500.0 F g−1 at a current density of 100 A g−1). Such excellent capacitive behavior is attributed to the synergetic effect between RuO2 and Co3O4, as well as the unique feature of heterostructure. Our results demonstrate that the simple method of electrospinning could also be extended to preparing other noble metal oxides@transition metal oxides heterogeneous structures with promising applications in the field of electrochemical energy storage.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21303046), the Research Fund for the Doctoral Program of Higher Education (No. 20130161120014), China Scholarship Council (File No. 201308430178), Hunan University Fund for Multidisciplinary Developing (No. 531107040762), Hunan Provincial Innovation Foundation for Postgraduate (No. 521293041).Notes and references
- M. R. Palacin, Chem. Soc. Rev., 2009, 38, 2565–2575 RSC.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- X. Yu, B. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044–1051 CrossRef CAS PubMed.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P.-L. Taberna, Science, 2006, 313, 1760–1763 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- S. Vivekchand, C. S. Rout, K. Subrahmanyam, A. Govindaraj and C. Rao, J. Chem. Sci., 2008, 120, 9–13 CrossRef CAS.
- J. Robertson, Mater. Today, 2004, 7, 46–52 CrossRef CAS.
- K. Chau, Y. Wong and C. Chan, Energy Convers. Manage., 1999, 40, 1021–1039 CrossRef CAS.
- D. Kalpana, N. Renganathan and S. Pitchumani, J. Power Sources, 2006, 157, 621–623 CrossRef CAS.
- J.-H. Sung, S.-J. Kim and K.-H. Lee, J. Power Sources, 2003, 124, 343–350 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- T.-S. Hyun, H. L. Tuller, D.-Y. Youn, H.-G. Kim and I.-D. Kim, J. Mater. Chem., 2010, 20, 9172–9179 RSC.
- X. Wang, X. Wang, W. Huang, P. Sebastian and S. Gamboa, J. Power Sources, 2005, 140, 211–215 CrossRef CAS.
- S. Chen, J. Zhu, X. Wu, Q. Han and X. Wang, ACS Nano, 2010, 4, 2822–2830 CrossRef CAS PubMed.
- X.-h. Xia, J.-p. Tu, Y.-j. Mai, X.-l. Wang, C.-d. Gu and X.-b. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646–15654 CAS.
- K. R. Prasad and N. Miura, Appl. Phys. Lett., 2004, 85, 4199–4201 CrossRef CAS.
- C.-C. Hu, K.-H. Chang, M.-C. Lin and Y.-T. Wu, Nano Lett., 2006, 6, 2690–2695 CrossRef CAS PubMed.
- W.-C. Fang, M.-S. Leu, K.-H. Chen and L.-C. Chen, J. Electrochem. Soc., 2008, 155, K15–K18 CrossRef CAS.
- Z. S. Wu, D. W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H. M. Cheng, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- J. H. Jang, S. Han, T. Hyeon and S. M. Oh, J. Power Sources, 2003, 123, 79–85 CrossRef CAS.
- W.-C. Chen, C.-C. Hu, C.-C. Wang and C.-K. Min, J. Power Sources, 2004, 125, 292–298 CrossRef CAS.
- H. Pang, J. Deng, J. Du, S. Li, J. Li, Y. Ma, J. Zhang and J. Chen, Dalton Trans., 2012, 41, 10175–10181 RSC.
- D.-Y. Youn, H. L. Tuller, T.-S. Hyun, D.-K. Choi and I.-D. Kim, J. Electrochem. Soc., 2011, 158, A970–A975 CrossRef CAS.
- Y. Li, K. Huang, D. Zeng, S. Liu and Z. Yao, J. Solid State Electrochem., 2010, 14, 1205–1211 CrossRef CAS.
- N. Krstajić and S. Trasatti, J. Electrochem. Soc., 1995, 142, 2675–2681 CrossRef.
- H. Jiang, J. Ma and C. Li, Chem. Commun., 2012, 48, 4465–4467 RSC.
- I. Kovalenko, D. G. Bucknall and G. Yushin, Adv. Funct. Mater., 2010, 20, 3979–3986 CrossRef CAS.
- P.-C. Chen, G. Shen, Y. Shi, H. Chen and C. Zhou, ACS Nano, 2010, 4, 4403–4411 CrossRef CAS PubMed.
- Y. Gao, S. Chen, D. Cao, G. Wang and J. Yin, J. Power Sources, 2010, 195, 1757–1760 CrossRef CAS.
- T. Zhu, J. S. Chen and X. W. Lou, J. Mater. Chem., 2010, 20, 7015–7020 RSC.
- J. Zhu, D. Lei, G. Zhang, Q. Li, B. Lu and T. Wang, Nanoscale, 2013, 5, 5499–5505 RSC.
- B. Lu, Y. Wang, Y. Liu, H. Duan, J. Zhou, Z. Zhang, Y. Wang, X. Li, W. Wang and W. Lan, Small, 2010, 6, 1612–1616 CrossRef CAS PubMed.
- Z. Wang, X. Liu, M. Lv, P. Chai, Y. Liu, X. Zhou and J. Meng, J. Phys. Chem. C, 2008, 112, 15171–15175 CAS.
- J. Zhu, Z. Xu and B. Lu, Nano Energy, 2014, 7, 114–123 CrossRef CAS.
- N. Chakroune, G. Viau, S. Ammar, L. Poul, D. Veautier, M. M. Chehimi, C. Mangeney, F. Villain and F. Fiévet, Langmuir, 2005, 21, 6788–6796 CrossRef CAS PubMed.
- Z.-S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H.-M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed.
- C. Gao, X. Li, B. Lu, L. Chen, Y. Wang, F. Teng, J. Wang, Z. Zhang, X. Pan and E. Xie, Nanoscale, 2012, 4, 3475–3481 RSC.
- Q. Qu, P. Zhang, B. Wang, Y. Chen, S. Tian, Y. Wu and R. Holze, J. Phys. Chem. C, 2009, 113, 14020–14027 CAS.
- X. Li, J. Shao, J. Li, L. Zhang, Q. Qu and H. Zheng, J. Power Sources, 2013, 237, 80–83 CrossRef CAS.
- P. Justin, S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2010, 114, 5203–5210 CAS.
- W. Pell and B. Conway, J. Electroanal. Chem., 2001, 500, 121–133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04884f |
| This journal is © The Royal Society of Chemistry 2016 |