Graphene aerogel-supported and graphene quantum dots-modified γ-MnOOH nanotubes as a highly efficient electrocatalyst for oxygen reduction reaction†
Abstract
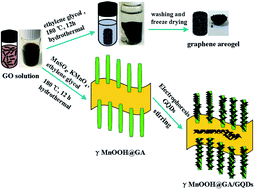
In this work, we demonstrate a facile strategy to synthesize a novel three-dimensional (3D) graphene aerogel-supported and graphene quantum dots-modified γ-MnOOH nanotubes as a highly efficient electrocatalyst. The structure, morphology, and chemical composition of γ-MnOOH@GA/GQDs are investigated by X-ray diffraction (XRD) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity of catalysts is discussed by cyclic voltammograms (CV), electrochemical impedance spectroscopy (EIS), and rotating disk electrode (RDE) measurements in O2-saturated 0.1 M KOH electrolyte. The γ-MnOOH@GA/GQDs hybrid exhibits more positive onset potential and half-wave potential, faster charge transfer, lower Tafel slope than that of γ-MnOOH@GA, GA and γ-MnOOH, and mainly undergoes a direct 4e− reaction pathway. Furthermore, its electrocatalytic performance is comparable with the commercial 20 wt% Pt/C, which is attributed to the unique 3D crumpled porous nanostructure of GA with large specific area and fast electron transport, and the synergic covalent coupling between the γ-MnOOH nanotubes and GA. More importantly, the GQDs structural defects can facilitate the adsorption of oxygen and charge transfer. As a highly efficient surface “sensitizer”, GQDs are modified on the γ-MnOOH surfaces to further boost the electrocatalytic property.

 Please wait while we load your content...
Please wait while we load your content...