A 1-D coordination polymer route to catalytically active Co@C nanoparticles†
Abstract
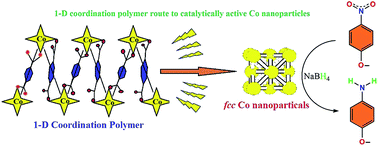
Pyrolysis of a 1-D polymeric cobalt(II) coordination complex ([Co(BDC)(Mim)2]n, H2BDC = benzenedicarboxylic acid; Mim = N-methylimidazole) results in the formation of carbon embedded fcc cobalt nanoparticle composites, Co@C. The as-prepared Co@C shows an agglomerated secondary structure with a highly embedded carbon shell comprising of cobalt nanoparticles of 20–100 nm. These Co@C particles show excellent catalytic activity in the reduction of nitrophenol to aminophenol, studied as a model reaction, and evolves as a promising candidate for the gas phase reduction process.

 Please wait while we load your content...
Please wait while we load your content...