Structure analysis of a glycosides hydrolase family 42 cold-adapted β-galactosidase from Rahnella sp. R3†‡
Abstract
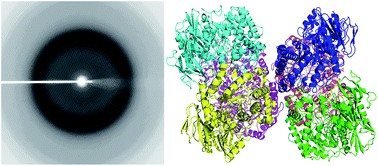
The β-galactosidase isolated from a psychrotrophic bacterium, Rahnella sp. R3 (R-β-Gal), exhibits high activity at low temperature and has potential in the dairy industry. R-β-Gal is a member of the glycoside hydrolases family 42 (GH42), and it forms a 225 kDa trimeric structure in solution. The crystals of R-β-Gal were acquired via hanging-drop vapor-diffusion method, and the X-ray crystal structure of the native R-β-Gal was determined at a 2.5 Å resolution. It is the first structure of a cold-adapted GH42 enzyme. In the crystallographic asymmetric unit, there were two homotrimers of the enzyme. Each monomer consists of three domains, an N-terminal catalytic domain which is a (β/α)8 barrel, a mixed β-sheet and α-helices domain, and a C-terminal β-sandwich domain. Two putative residues might be involved in catalysis, a proton donor E157 and a nucleophile E314, were superimposed well with the catalytic residues of other β-galactosidases. Site-directed mutagenesis targeting these residues abolished the activity of the enzyme. Structure and sequence comparison of R-β-Gal with two mesophilic β-gals and a thermophilic β-gal indicated that intramolecular force and higher structural flexibility might result in the cold-adaptation of R-β-Gal.

 Please wait while we load your content...
Please wait while we load your content...