Transcriptome-guided discovery and functional characterization of two UDP-sugar 4-epimerase families involved in the biosynthesis of anti-tumor polysaccharides in Ornithogalum caudatum†
Sen Yin and
Jian-Qiang Kong*
Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College (State Key Laboratory of Bioactive Substance and Function of Natural Medicines & Ministry of Health Key Laboratory of Biosynthesis of Natural Products), Beijing, 100050, China. E-mail: jianqiangk@imm.ac.cn
First published on 6th April 2016
Abstract
UDP-glucose 4-epimerase (UGE) and UDP-xylose 4-epimerase (UXE), two important UDP-sugar 4-epimerases, are well known to be essential for de novo biosynthesis of UDP-D-galactose and UDP-L-arabinose, two universal sugar donors for the formation of four galactose- and arabinose-containing anticancer polysaccharides in Ornithogalum caudatum. However, very little is known about their cDNA sequences. Furthermore, the functional significance of the two epimerases in the biosynthesis of these anticancer polysaccharides in O. caudatum had not been documented. Here, we presented a full characterization of UGE and UXE, which were deemed to be responsible for anticancer polysaccharides biosynthesis in O. caudatum. Specifically, a transcriptome-guided search for the two epimerase genes in O. caudatum was first performed in the present study. A total of 4 unigenes sharing high sequence identity with UDP-sugar 4-epimerases were retrieved from transcriptome assembly. Four full-length cDNAs encoding UDP-sugar 4-epimerases, including two UGE-like and two UXE-like genes, were then isolated by reverse transcription polymerase chain reaction (RT-PCR) from O. caudatum. Bio-informatic analysis indicated the two UDP-sugar 4-epimerase families shared two common conserved domains, namely an N-terminal GxxGxxG motif and a catalytic Ser/Thr-Tyr-Lys triad. A phylogenetic analysis revealed the two members in the same UGE family could be classified into two subgroups, revealing their divergently functional significance. These candidate isoenzymes were screened by functional expression in E. coli individually as standalone enzymes. Two UGE-like cDNAs were identified to be bona fide genes, exhibiting both UGE and UXE activities. To further explore the possible role of these epimerase proteins in polysaccharides biosynthesis, transcript profiles of the four genes were subsequently examined by real-time quantitative PCR in various O. caudatum tissues. OcUGE1, OcUGE2 and OcUXE1 were therefore assumed to be responsible for the biosynthesis of the four galactose- and arabinose-containing polysaccharides due to their expression profiles in O. caudatum. Taken together, these data provide further comprehensive knowledge for polysaccharides biosynthesis in O. caudatum and broaden the potential application of UGE in metabolic engineering or synthetic biology as a potential gene part.
1. Introduction
UDP-glucose 4-epimerase (UGE, also named UDP-galactose 4-epimerase, EC 5.1.3.2), one of the UDP-sugar 4-epimerases, catalyzes the interconversion of UDP-D-glucose (UDP-D-Glc) and UDP-D-galactose (UDP-D-Gal) via a UDP-4-keto-hexose intermediate (Fig. 1).1–5 Also, many UGEs in vascular plants were found to exhibit UDP-xylose 4-epimerase (UXE, EC 5.1.3.5) activity (Fig. 1), interconverting UDP-D-xylose (UDP-D-Xyl) and UDP-L-arabinose (UDP-L-Ara).6 Moreover, a UGE from barley (Hordeum vulgare L.) was reported to have a very low UDP-N-acetylglucosamine 4-epimerase (UGlcNAcE, EC 5.1.3.7) activity, catalyzing the interconversion between UDP-N-acetylglucosamine (UDP-GlcNAc) and UDP-N-acetylgalactosamine (UDP-GalNAc).7 These resultant UDP-sugars by the action of UGEs can act as building blocks of polysaccharides, glycoproteins and glycosides. The substrates promiscuity of UGE is, therefore, deemed to play important roles in metabolism of carbohydrates, providing precursors for polysaccharides biosynthesis.8 Furthermore, UGE was reported to play vital roles in plant growth and development.9–11 Besides formation of UDP-D-Gal through de novo pathway, UGE also converts excess UDP-D-Gal and UDP-L-Ara derived from salvage pathway into their respective 4-epimers.5 Taken together, these data indicated the central role of UGE in regulation of carbohydrate partitioning in vascular plants.12 Unlike UGE, another UDP-sugar 4-epimerase, UXE, has strict substrate specificity and only catalyzes the interconversion of UDP-D-Xyl and UDP-L-Ara (Fig. 1). UXE also plays key role in carbohydrate partitioning, plant growth and development.13 In general terms, it can be concluded that UGE and UXE should be important in the composition of the sugar nucleotide pools. | ||
| Fig. 1 Interconversion reactions between both UDP-D-Glc and UDP-D-Gal, and UDP-D-Xyl and UDP-D-Ara catalyzed by UGEs and UXEs. | ||
The two nucleotide sugar 4-epimerases are demonstrated to widely exist in varied organisms, including plants,14,15 human,16 fungi17,18 and prokaryotes.19,20 In plants, UGE is found to distribute in Arabidopsis thaliana,8,11,12,14,21 barley (Hordeum vulgare L.),7 pea (Pisum sativum L.),6,22 rice10 and wheat,23 while UXE is reported to occurs in barley,15 Arabidopsis thaliana13 and wheat.24 Although wide distribution in plants, the genes encoding UGE and UXE from Ornithogalum caudatum had still not be documented.
O. caudatum, an annual herb originally distributed in southern Africa and introduced to ancient China, was known in Chinese folk medicine as exhibiting anticancer, antimicrobial and anti-inflammatory activities.25 OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3, isolated from O. caudatum, are four galactose- and arabinose-containing polysaccharides.26 These polysaccharides exhibit significant anticancer action, suggesting their potential as anticancer drugs. Monosaccharide components characterization revealed these polysaccharides consist primarily of glucose, galactose, arabinose, xylose, glucuronic acid and galacturonic acid. Besides glucose, galactose and arabinose are also two main components of the four polysaccharides, accounting for about 20 and 10% (molar ratio) of the total sugars, respectively.26 The incorporation of galactose and arabinose into polysaccharides requires the addition from their respective nucleotide-activated precursors, typically UDP-D-Gal and UDP-L-Ara.2,3,5 Thus, the biosynthesis of the four polysaccharides requires at least enzymes for the synthesis of each nucleotide-activated sugar precursor. While biochemical aspects of these nucleotide sugars biosynthetic pathways are reasonably well understood,2,3,5 very little is known about the cDNA isolation and functional significance of pathway enzymes, including UGE and UXE, involved in UDP-D-Gal and UDP-L-Ara biosynthesis in O. caudatum.
As a first step to investigate the biosynthesis of galactose- and arabinose-containing polysaccharides in O. caudatum, a transcriptome-guided gene discovery and functional characterization of two UDP-sugar 4-epimerase families was performed in the present study. Specifically, a total of four full-length cDNAs, including two UGE genes and two UXE genes were isolated for the first time from O. caudatum based on a transcriptome-wide search. These candidate isoforms were then screened by the functional expression in E. coli individually as standalone enzymes. Results showed two UGE cDNAs were bona fide genes and encoded UDP-glucose 4-epimerase. Most important, the present investigation preliminarily revealed the involvement of OcUGE1, OcUGE2 and OcUXE1 in biosynthesis of the four polysaccharides in O. caudatum. Discovery and characterization of UGE family will help to comprehensive understanding the anticancer polysaccharides biosynthesis in O. caudatum.
2. Materials and methods
2.1. Chemicals and enzymes
Nucleotide sugars UDP-D-xylose, UDP-L-arabinose and UDP-D-galacturonic acid (UDP-D-GalA) are from CarboSource Services, University of Georgia, Athens. UDP-D-glucose, UDP-D-galactose, UDP-D-glucuronic acid (UDP-D-GlcA), NAD+, NADP+, NADH and NADPH were obtained from Sigma-Aldrich Co. LLC (St. Louis, MO, United States). In-Fusion® HD Cloning Kit and restriction enzymes were purchased from Takara Shuzo Co. Ltd (Kyoto, Japan). KOD Plus Taq DNA polymerase was purchased from Toyobo Co. Ltd (Osaka, Japan). The other fine chemicals (suppliers) were listed as follows. Acetonitrile (Sigma-Aldrich Co. LLC) was used for HPLC analysis. IPTG (isopropyl β-D-thiogalactoside) was purchased from Sigma-Aldrich Co. LLC and applied to induce protein expression in Escherichia coli.2.2. Strains and plasmids
pEASY®-Blunt vector was from TransGen Co. Ltd (Beijing, China). The E. coli strain Trans1-T1, Transetta (DE3) and BL21 (DE3) (TransGen Co. Ltd) were used as a bacterial host for recombinant plasmid amplification and enzyme expression, respectively. The strain was grown in Luria-Bertani medium (10 g L−1 Bacto-Tryptone, 5 g L−1 Bacto-yeast extract, 10 g L−1 NaCl) supplemented with ampicillin (100 μg mL−1) when required for selection.The expression vector pET-28a (+) was from Novagen (Madison, USA) and used for heterologous expression. The plasmids and strains used in this study are provided in ESI Table S1.†
2.3. Plant materials
Plant cultivation of O. caudatum was performed as described previously.27,28 Fresh tissue samples of roots, leaves, bulbs, flowers, bulblets, alabastrums were then collected from a 2 year-old O. caudatum for analysis of the expression profiles of UGE and UXE genes. Moreover, the sterile bulbs of O. caudatum inoculated on 6, 7-V medium29 were used as the start material for total RNA isolation, thereby providing templates for cDNA isolation and expression analysis of UGE and UXE genes.2.4. RNA-Seq data analysis
O. caudatum transcriptome was analyzed by high-throughput RNA sequencing (RNA-Seq) previously in our laboratory.27,28,30–32 The resulting transcriptome data were further analyzed in the present study, aiming at retrieving nucleotide sequences showing high identity with UDP-sugar 4-epimerase. Specifically, these resulting unigenes based on transcriptome assembly were aligned by BLASTX to protein databases like NCBI nr, Swiss-Prot, KEGG and COG (e-value < 0.00001), and aligned by BLASTN to nucleotide databases nt (e-value < 0.00001), resulting in proteins with the highest sequence similarity with the given unigenes along with their functional annotations. These unigenes showing high identity with UGE and UXE genes were selected for further investigation.2.5. Bioinformatics analysis
Varied bioinformatics tools were applied to analyze the obtained candidate unigenes. These tools included Open reading frame (ORF) Finder (http://www.ncbi.nlm.nih.gov/grof/orfig.cgi) for ORF identification and ProtParam tool (http://web.expasy.org/protpatam/) for physicochemical parameters evaluation of proteins. Moreover, TMHMM (http://www.cbs.dtu.dk/services/TMHMM/), SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/), TargetP 1.1 Server (http://www.cbs.dtu.dk/services/TargetP/) and PROSITE tools (http://prosite.expasy.org/) were used for the prediction of the transmembrane helices, cleavage sites of signal peptides, subcellular locations and functional sites of the interest proteins, respectively. Protein multiple sequence alignment was performed using ClustalX. A phylogenetic tree was constructed using the neighbor-joining method with the MEGA5.1 program. The reliability of the tree was measured by bootstrap analysis with 1000 replicates.2.6. cDNA isolation and functional characterization of OcUGE gene family
Since the assembled unigenes were products of de novo assemblies, they were considered prone to error. To confirm the authenticity of these unigenes, experimental verifications were performed by designing gene-specific primers for these full-length sequences encoding UGE and verifying the identity of amplified products by sequencing of the PCR amplimers. All the oligonucleotides used for DNA manipulation are described in the ESI Table S2.†Total RNA was extracted from the sterile bulbs of O. caudatum, using an RNeasy Plant Mini Kit (Qiagen). First-strand cDNA synthesis was carried out using 1 μg of total RNA with primer oligo (dT)20 according to the protocol of ReverTra Ace (TOYOBO). The amplification of OcUGE cDNAs was performed by a nested PCR method using KOD Plus Taq polymerase and gene-specific primers (Table S2†). The amplified full-length cDNAs each were inserted into the pEASY®-Blunt vector to generate pEASY-OcUGEs for sequencing, respectively.
After sequence verifications, OcUGE1 or OcUGE2 was inserted into EcoRI and Hind III linearized pET-28a (+) to generate a recombinant vector for heterologous expression using In-Fusion technology as previously described.27,28,30–32 Successful gene cloning was verified by digestion checks, and the absence of undesired mutation introduced during PCR was verified by direct nucleotide sequencing.
The expression plasmid pET28a-OcUGE was transformed into the E. coli strain Transetta (DE3) grown in LB media containing 50 μg mL−1 kanamycin and 35 μg mL−1 chloromycetin. Expression of the recombinant protein was induced at an OD600 of 0.6–1.0 by addition of IPTG to a final concentration of 0.5 mM. After shaking at 25 °C overnight, the induced cells were harvested by centrifugation (7500g, 2 min) at 4 °C. The pellets were then either stored at −80 °C or used directly.
To prepare a crude protein extract from E. coli, frozen cells obtained from a 100 mL culture were re-suspended in 5 mL of 50 mM sodium phosphate (pH 8.0) containing 5 mM imidazole and 300 mM NaCl. A small part of pellets (derived from 1 mL culture) were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. After verification of successful expression, the rest cells were re-suspended in lysis buffer (pH 8.0, 20 mM sodium phosphate) and lyzed by sonication. The resulting lysate was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min. The obtained supernatant was passed through a 0.2 μm pore-size filter to remove E. coli debris and other contaminants. Purification of the native recombinant protein from crude extracts was carried out with Ni-NTA agarose columns according to the manufacture's protocol (Qiagen).
000g for 15 min. The obtained supernatant was passed through a 0.2 μm pore-size filter to remove E. coli debris and other contaminants. Purification of the native recombinant protein from crude extracts was carried out with Ni-NTA agarose columns according to the manufacture's protocol (Qiagen).
Unless otherwise indicated, the standard 50 μL assay system contained 50 mM sodium-phosphate (pH 7.0), 1 mM UDP-D-sugars, 10 μg purified protein. The reaction was allowed to proceed for 30 min at 30 °C, and terminated by the addition of 50 μL chloroform. The mixture was vortexed and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. The resulting upper aqueous phase was then collected and the products were unambiguously determined by HPLC-UV, HPLC-MS and 1H NMR as described previously.28,30 It is noteworthy that nucleotides and nucleotide sugars were detected on an UV detector at 261 nm.
000g for 10 min. The resulting upper aqueous phase was then collected and the products were unambiguously determined by HPLC-UV, HPLC-MS and 1H NMR as described previously.28,30 It is noteworthy that nucleotides and nucleotide sugars were detected on an UV detector at 261 nm.
2.7. cDNA isolation and prokaryotic expression of OcUXE gene family
The full-length ORFs of candidate UXE genes were isolated from O. caudatum by nested PCR using the gene-specific primers (Table S2†). Vectors construction, sequences verification, proteins expression and purification were performed using the same procedure as described above. To test OcUXEs activity, 1 mM UDP-D-Xyl was added into 50 μL sodium phosphate buffer (50 mM, pH 7.0) containing 5 μg of purified protein. The reaction was kept at 30 °C for 30 min and terminated by addition of 50 μL chloroform. The reaction products were monitored by HPLC-DAD as described above.2.8. Enzymatic properties of OcUGE gene family
The phylogenetic analysis of OcUGE1 and OcUGE2 showed their divergent classification in two subgroups, suggesting their diverse functions in planta. Therefore, to get a comprehensive knowledge of OcUGE1 and OcUGE2, a systematical investigation about the qualitative and quantitative biochemical difference between OcUGE1 and OcUGE2 was performed.The biochemical properties were tested in a variety of buffers, at different temperatures and diverse additives. The optimum pH and temperature evaluation and kinetic analysis of OcUGE1 and OcUGE2 proteins were carried out as described previously.28,30–32 Briefly speaking, pH profile of recombinant OcUGE proteins was determined in varied buffers, including acetate buffer (pH 4–5), sodium phosphate buffer (pH 6–8) and Tris–HCl buffer (pH 9–11). Assays were performed at a constant temperature of 30 °C for 30 min using UDP-D-Gal and UDP-D-Xyl as the substrates, respectively.
For determination of the optimal temperature, reactions were performed in 100 mM sodium phosphate buffer (pH 8.0) between 0 and 50 °C with a 10 °C interval.
Varied additives (CaCl2, MgSO4, MnCl2, EDTA, 10 mM; NH4Cl, 50 mM; NAD+, NADP+, NADPH, NADH, 1 mM) were added to sodium phosphate buffer (100 mM, pH 8.0) to investigate their influence on the UXE activity of OcUGEs. Assays were performed using UDP-D-Xyl as the substrate at 30 °C for 30 min. The formation of UDP-L-Ara was monitored by HPLC.
Kinetic analysis of the recombinant OcUGE proteins was performed in 50 μL sodium phosphate buffer (100 mM, pH 8.0) containing varied substrates and purified OcUGE recombinant proteins. Assays for individual substrates were performed in triplicate at 30 °C for 30 min. Kinetic constants values were determined from Lineweaver–Burk plots. All kinetic assays were performed in triplicate and controls without enzymes or substrates were included.
2.9. Tissue-specific expression analysis of OcUGE proteins by real-time quantitative PCR
The analysis of transcript levels of specific genes is important for the characterization of gene function in planta. Real-time quantitative PCR (RT-qPCR) is therefore applied to analyze the expression profiles of OcUGEs and OcUXEs in O. caudatum with the aim to verify the genes involved in anticancer polysaccharides biosynthesis. Specifically, total RNA was isolated from various tissues including roots, leaves, bulbs, flowers, bulblets, alabastrum and sterile bulbs of O. caudatum using an RNeasy Plant Mini Kit (Qiagen). One μg of total RNA was used for first-strand cDNA synthesis according to the protocol of ReverTra Ace (TOYOBO). Real-time quantitative PCR (RT-qPCR) was performed as described previously.27 All reactions were carried out in triplicate, and the data were analyzed using the Lightcycler® 480 software.3. Results and discussion
3.1. Transcriptome analysis of O. caudatum unigenes
The transcriptome is the complete set of transcripts within a cell at some particular state.33,34 Transcriptome sequencing is a high-throughput approach and can yield a tremendous amount of sequences in each run, far greater than that produced by traditional techniques. Transcriptome sequencing, therefore, can greatly accelerate full-length genes isolation. In the previous study, a total of 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 unigenes with an average length of 520 bp were acquired from transcriptome de novo assembly of O. caudatum.28 In this investigation, these unigenes were first subjected to Basic Local Alignment Search Tool (BLAST X and BLAST N) against NCBI protein and nucleotide databases for functional annotation. A total of four unigenes showing high identity with UDP-sugar epimerase were thus retrieved from RNA-Seq data of O. caudatum. Their transcript IDs are listed in Table 1. These unigenes were further analyzed by BLAST X and ORF Finder for their ORF identification. Results revealed every unigenes contained a full-length ORF and partial 5′-untranslated region (UTR) and 3′-UTR sequences. In particular, unigene 9569 was 1797 bp long with an ORF of 1251 bp encoding 416 amino acids and consisted of a partial 5′-UTR of 188 bp and 3′-UTR of 358 bp. Unigene 9803 is 1651 bp long and contains a 1083 bp ORF encoding a protein of 360 amino acids with an ATG at position 153 and the stop codon TGA at position 1233. The length of partial 5′-UTR and 3′-UTR in unigene 9803 was 152 and 416 bp, respectively. The other two unigenes 33
180 unigenes with an average length of 520 bp were acquired from transcriptome de novo assembly of O. caudatum.28 In this investigation, these unigenes were first subjected to Basic Local Alignment Search Tool (BLAST X and BLAST N) against NCBI protein and nucleotide databases for functional annotation. A total of four unigenes showing high identity with UDP-sugar epimerase were thus retrieved from RNA-Seq data of O. caudatum. Their transcript IDs are listed in Table 1. These unigenes were further analyzed by BLAST X and ORF Finder for their ORF identification. Results revealed every unigenes contained a full-length ORF and partial 5′-untranslated region (UTR) and 3′-UTR sequences. In particular, unigene 9569 was 1797 bp long with an ORF of 1251 bp encoding 416 amino acids and consisted of a partial 5′-UTR of 188 bp and 3′-UTR of 358 bp. Unigene 9803 is 1651 bp long and contains a 1083 bp ORF encoding a protein of 360 amino acids with an ATG at position 153 and the stop codon TGA at position 1233. The length of partial 5′-UTR and 3′-UTR in unigene 9803 was 152 and 416 bp, respectively. The other two unigenes 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 and 67
897 and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 were 1930 and 1473 bp in length and encoded polypeptides of 418 and 352 amino acid residues, respectively. The length of their ORFs, 5′-UTRs and 3′-UTRs was listed in detail in Table 1. Further analysis showed the four unigenes belong to two UDP-sugar epimerase families. Concretely speaking, unigenes 9569 and 33
545 were 1930 and 1473 bp in length and encoded polypeptides of 418 and 352 amino acid residues, respectively. The length of their ORFs, 5′-UTRs and 3′-UTRs was listed in detail in Table 1. Further analysis showed the four unigenes belong to two UDP-sugar epimerase families. Concretely speaking, unigenes 9569 and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 belong to UDP-D-xylose epimerase family, while unigenes 9803 and 67
897 belong to UDP-D-xylose epimerase family, while unigenes 9803 and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 are members of UDP-D-glucose epimerase family.
545 are members of UDP-D-glucose epimerase family.
| Unigene ID | Length (bp) | Sequence structure (bp) | ||
|---|---|---|---|---|
| 5′-UTR (partial) | ORF | 3′-UTR (partial) | ||
| 9569 | 1797 | 188 | 1251 | 358 |
| 9803 | 1651 | 152 | 1083 | 416 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897 |
1930 | 294 | 1257 | 379 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 545 |
1473 | 86 | 1059 | 328 |
3.2. cDNA isolation and sequences analysis of OcUGE and OcUXE gene families
To confirm the identity of these four unigenes, cDNA isolation from O. caudatum was subsequently performed. More specifically, these predicted cDNA sequences were isolated from O. caudatum cDNA library directly by nested PCR using the gene-specific primers (Table S2†), first and then, these resultant ORFs each were inserted to pEASY®-Blunt vector for sequencing. Sequencing verified that these cDNA sequences were identical with the results from transcriptome sequencing, which means the real genes in planta. Therefore, these ORFs derived from unigenes 9569, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897, 67
897, 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 and 9803 were designated as OcUXE1, OcUXE2, OcUGE1 and OcUGE2 and deposited in the GenBank database, respectively (Table 2). Hence, both OcUGE and OcUXE are encoded by a small gene family, which is consistent with previous reports.13–15,35 Although the significance of existence of multiple UGE or UXE isoforms in the same species is unclear, the retention of paralogs might be beneficial in selective terms.12 The four sequences were subsequently analyzed by bioinformatics tools, aimed at successfully heterologous expression and functional characterization.
545 and 9803 were designated as OcUXE1, OcUXE2, OcUGE1 and OcUGE2 and deposited in the GenBank database, respectively (Table 2). Hence, both OcUGE and OcUXE are encoded by a small gene family, which is consistent with previous reports.13–15,35 Although the significance of existence of multiple UGE or UXE isoforms in the same species is unclear, the retention of paralogs might be beneficial in selective terms.12 The four sequences were subsequently analyzed by bioinformatics tools, aimed at successfully heterologous expression and functional characterization.
| GenBank accession no. | Length (bp) | Molecular mass of deduced protein (kDa) | Theoretical pI | Amino acid identity/query cover (100%) | Predicted membrane topology | |
|---|---|---|---|---|---|---|
| OcUXE1 | KU664040 | 1251 | 45.4 | 9.05 | 100/100 | YES, type I membrane protein |
| OcUXE2 | KU664041 | 1257 | 46.2 | 6.93 | 69/94 | YES, type I membrane protein |
| OcUGE1 | KU664038 | 1059 | 38.9 | 7.07 | 80/98 | NO |
| OcUGE2 | KU664039 | 1083 | 39.7 | 8.35 | 100/100 | NO |
OcUGE1 and OcUGE2 encode cytoplasmic soluble protein of 352 and 360 amino acids respectively. This notion is consistent with previous reports, where UGE genes are deemed to encode enzymes that lack transmembrane peptides and locate in the cytosol.6,7,14 OcUXE1 and OcUXE2 encode predicted polypeptide consisting of 416 and 418 amino acids, respectively. PSORT program analysis indicates both OcUXE1 and OcUXE2 likely encode typeI membrane-anchored proteins with respective membrane-spanning domain of amino acids. The membrane-bound UXEs were also found in Arabidopsis thaliana13 and barley (Hordeum vulgare).15
The amino acid alignment of the UGE and UXE isoforms in O. caudatum is shown in Fig. 2A. The results indicated that both OcUXEs contained about 60 more amino acid residues than OcUGE1 and OcUGE2 at the N-terminus, which were deemed to be characteristic of membrane-bound proteins (Fig. 2A). All of the four amino acid sequences have several conserved fingerprint sequences, including an N-terminal GxxGxxG (x refers to any amino acid) motif that is characteristic of βαβαβ Rossmann NAD(P)-binding proteins,36,37 and a characteristic and highly conserved Thr/Ser-Tyr-Lys triad (Fig. 2A).38,39 GxxGxxG, a motif stabilizing NAD(P)-binding Rossmann folds and binding of the nucleotide cofactor to the domain,36 is strictly conserved among the four epimerases (Fig. 2A). The catalytic Ser/Thr-Tyr-Lys triad is a fingerprint motif among the dehydrogenase/reductase superfamily and involved in oxidoreductase activity. In the triad, Lys and Tyr are present in the completely conserved YxxxK motif (x refers to any amino acid) (Fig. 2A). Instead, the third residue in the triad varied between UGEs and UXEs. In UGEs, the third residue is Ser, while in UXEs, the third residue is changed to Thr, which is consistent with the previous reports (Fig. 2A).13,15 Therefore, it is able to preliminarily distinguish between UGE and UXE based on third amino acid residues in Thr/Ser-Tyr-Lys triad.
Phylogenetic relationships among UGEs, UXEs, UGlcAEs, UXSs (UDP-xylose synthase, EC 4.1.1.35) and UAXSs (UDP-D-apiose/UDP-D-xylose synthase) from varied origins were analyzed. As illustrated in Fig. 2B, the five protein families are clustered clearly distinct clades, suggesting their divergently functional significance in vivo. The UGE clade can be further divided into two separated subgroups, UGE1 and UGE2. Both UGE1 and UGE2 clades contain multiple genes encoding UGE derived from varied monocotyledons and dicotyledons. OcUGE1, together with AtUGE1 and AtUGE3 of Arabidopsis thaliana and other UGEs from Pisum sativum, Oryza sativa and Hordeum vulgare formed the UGE1 clade. The UGE2 clade contained OcUGE2 and other UGEs derived from different plant species like Arabidopsis thaliana, Oryza sativa and Hordeum vulgare. The notion of presence of two subgroups in UGE family was also described previously.6,12,15 The separation of OcUGE1 and OcUGE2 probably reflects a difference in their in vivo functions. Moreover, this separation of UGE1 and UGE2 should predate the separation of dicotyledons and monocotyledons. Unlike the divergent tendency of UGE family, OcUXE1 and OcUXE2, together with other UXEs from other origins are clustered a single clades, suggesting their conserved functions in the course of the evolution of higher plants (Fig. 2B).
3.3. Heterologous expression and functional characterization of OcUGE gene family
Based on sequence similarities, we were able to identify the genes for the biosynthesis of UDP-D-galactose and UDP-L-arabinose. However, direct biochemical evidence to confirm these annotations remained to be established. Thus, these four candidate isoenzymes were screened by functional expression in E. coli individually as standalone enzymes. Generally speaking, two full-length OcUGEs were introduced into E. coli Transetta (DE3) in the form of plasmid pET28a-OcUGE, first and then, induced to express as a His6-OcUGE fusion protein, respectively. OcUGE1 and OcUGE2 were expressed in soluble proteins as visualized by SDS-PAGE analysis (Fig. 3). The His6-tagged recombinant OcUGE1 and OcUGE2 proteins were subsequently column-purified to near homogeneity (Fig. 3). And then, these resulting purified proteins each were incubated with UDP-D-Glc, UDP-D-Gal, UDP-D-Xyl, UDP-L-Ara, UDP-D-GlcA or UDP-D-GlcNAc to evaluate their epimerase activity, respectively. | ||
| Fig. 3 SDS-PAGE analysis of recombinant OcUGE1 (a) and OcUGE2 (b). The arrows indicate the expressed recombinant proteins. | ||
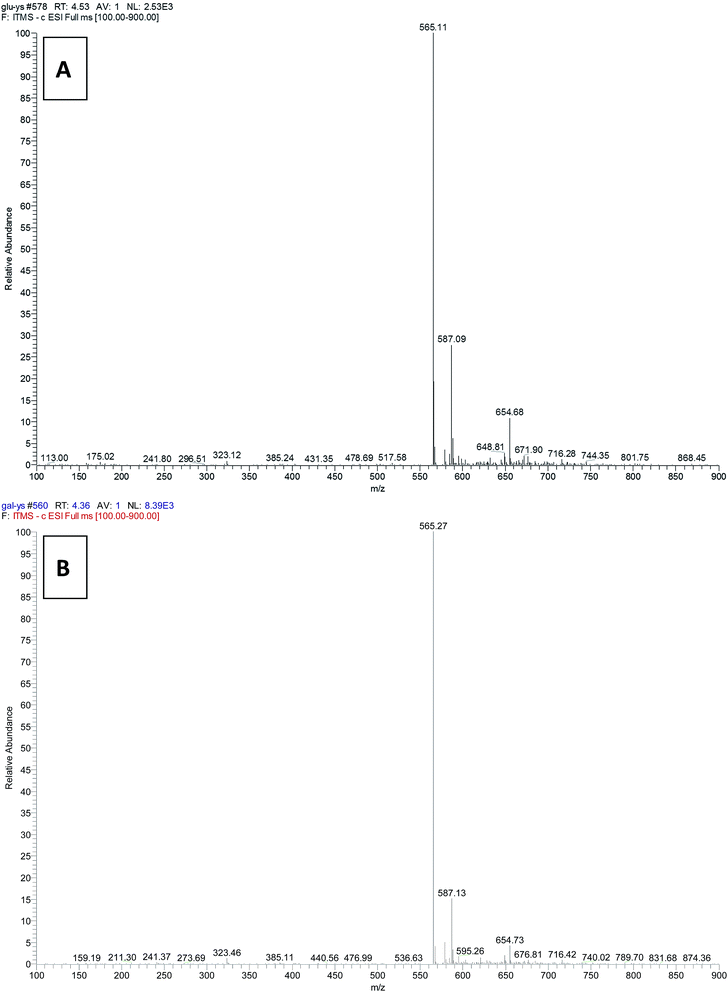
The UDP-glucose 4-epimerase activity of OcUGEs was monitored by a combination of HPLC and LC-MS. When substrate UDP-D-Glc was incubated with a recombinant OcUGE protein, a new peak with the same retention time and UV spectra as the authentical UDP-D-Gal, was detected in the HPLC profile (Fig. 4a). To further verify that the newly formed reaction products represented exactly UDP-D-Gal, the UDP-D-Gal standard was co-injected with the reaction mixture under the HPLC-based assay. Only a new peak with no shouldering was found by HPLC analysis, suggesting UDP-D-Gal is produced from epimerization reaction of UDP-D-Glc in both of OcUGEs (Fig. 4c). Finally, the identity of UDP-D-Gal was confirmed by mass spectrometry. The newly formed products exhibited an ion at m/z 565.27 (Fig. 5), which is identical to that of UDP-D-Gal standard. Taken together, these data unambiguously confirmed that the newly formed product catalyzed by OcUGE proteins from UDP-D-Glc is UDP-D-Gal. Moreover, both of the two enzymes also catalyzed the reverse reaction from UDP-D-Gal to UDP-D-Glc (Fig. 4a). Specifically, when UDP-D-Gal was added to the reaction mixture containing purified OcUGEs, a new product, which was characterized to be UDP-D-Glc by combination of HPLC (Fig. 4a and c) and LC-MS (Fig. 5), was detected in HPLC profile. These data unambiguously testified that both OcUGE proteins have UDP-glucose 4-epimerase activity.
To identify the UDP-xylose 4-epimerase action of the two enzymes, the substrate UDP-D-xylose or UDP-L-arabinose was incubated with purified recombinant OcUGE proteins in the forward and reverse reactions, respectively. In the forward reaction, when UDP-D-Xyl was incubated with recombinant OcUGEs, a newly formed product with the same retention time as the standard UDP-L-Ara was detected (Fig. 4b). LC-MS analysis of the newly formed product displayed its [M − H]− ions at m/z 535.16, corresponding to the calculated mass for UDP-L-Ara. The product of the OcUGEs reactions was further analyzed by 1H-NMR. It was concluded from the 1H-NMR parameters that the OcUGE1 reaction product was UDP-L-Ara (Fig. 6). Moreover, both of the two enzymes also catalyzed the reverse reaction from UDP-L-Ara to UDP-D-Xyl (Fig. 4b). LC-MS analysis of the newly formed product displayed its [M − H]− ions at m/z 535.0109, corresponding to the expected mass for UDP-D-Xyl. These results unambiguously indicated their UXE activity of OcUGE1 and OcUGE2.
However, when UDP-D-GlcA or UDP-D-GlcNAc was added to the reaction system containing OcUGEs, no products were detected (data not shown). Additionally, the UDP-GlcNAc 4-epimerase activity of the two OcUGEs was not performed due to unavailability of substrates UDP-GlcNAc and UDP-GalNAc.
Taken together, the data revealed that OcUGE1 and OcUGE2 are bifunctional UDP-glucose 4-epimerase catalyzing interconversions between both UDP-D-Glc and UDP-D-Gal, and UDP-D-Xyl and UDP-L-Ara. This fact, together with previous reports,6,14 collectively suggests that UGEs with UDP-xylose 4-epimerase activity are conserved in vascular plants.
UGE is reported to be an important building block of glycosyl donors for glycosides production in metabolic engineering and synthetic biology.40–42 UGE and glycosyltransferase specific for quercetin and UDP-L-Ara were co-expressed in E. coli, which resulted in an engineered factory producing about 160 mg L−1 quercetin-3-O-arabinoside.42 Also, in another engineered E. coli containing UGE and glycosyltransferase, 280 mg L−1 quercetin-3-O-galactoside can be obtained.40 Obviously, the yields of galactosides and arabinosides produced by these engineered cells were far from industrial requirement. The low yields of glycosides were partly due to the suboptimal performance of the foreign pathways containing UGE in microbial cells.
It is well known that the performance of a foreign pathway in engineered cells is dictated by the kinetic properties of its enzymatic components.43,44 Variation of these kinetic properties may be achieved by components replacement with suitable enzyme homologs, which were obtained either by mutagenic experiments or the well-characterized variants saved in the public databases. Our successful characterization of OcUGEs therefore provides one more selection for a component replacement in a foreign pathway containing UGE, thereby increasing the possibility of pathway optimization and further yields improvement.
3.4. Heterologous expression and functional characterization of OcUXE genes family
Two full-length OcUXE genes were cloned into E. coli vector pET-28a (+). The resulting two plasmids pET28a-OcUXE1 and pET28a-OcUXE2 were transformed into E. coli Transetta (DE3) to be induced for soluble expression, respectively. However, there is undetectable expression of OcUXE1 and OcUXE2. The presence of variable N-terminal extension regions is assumed to be the main reason of no detectable expression of these genes. Therefore, the coding DNA sequences on and before the predicted trans-membrane region were excluded from the respective OcUXE1 and OcUXE2 to yield truncated UXEs (tOcUXE1 and tOcUXE2). These truncated cDNAs were subcloned into the expression vector pET-28a (+) again and then induced to express in E. coli by IPTG as described above. A massive inclusion body representing tOcUXE1 and tOcUXE2 were produced as visualized in SDS-PAGE results (data not shown). Inclusion body formation during the course of heterologous expression of recombinant tOcUXE1 and tOcUXE2 was indicative of their inability to form nature or correct tertiary structure. This protein misfold often requires the assistance of folding modulators, like molecular chaperones. It is widely recognized that co-expression of molecular chaperones can favor on-pathway folding and that in at least some cases this leads to increased production of active proteins.45,46 The co-expressions of recombinant plasmid pET28atOcUXE1 and chaperone plasmid pGro7 (Takara, Dalian, China), and pET28atOcUXE2 and another chaperone plasmid pKJE7 were, therefore, performed in E. coli strain BL21 (DE3) for soluble expression. As depicted in Fig. S1,† two intense bands each with an apparent molecular mass of 38 kDa were determined by SDS-PAGE detection, suggesting the successful expression of a soluble OcUXE1 and OcUXE2 proteins (Fig. S1†). The crude extracts from E. coli expressing truncated OcUXE1 and OcUXE2 each were then used to assay nucleotide sugar interconversion activity. The crude extracts were incubated with UDP-D-Glc, UDP-D-Gal, UDP-D-Xyl, UDP-L-Ara, UDP-D-GlcA or UDP-D-GlcNAc, respectively. However, there is no detectable product yield (data not show). The detail reason is not clear, and we presumed that decreased stability caused by N-terminal truncation may be the main reason of no activity of these truncated versions of OcUXEs. As such, a further study is required for comprehensive knowledge of OcUXEs.3.5. Biochemical properties of OcUGE1 and OcUGE2
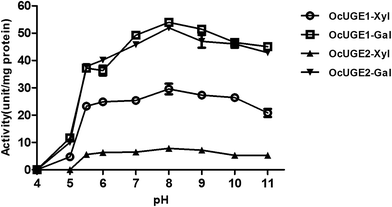
OcUGE is encoded by a small gene family, containing at least two members. The apparent genetic redundancy was also present in other nucleotide sugar interconversion enzymes.27 The functional significance of biochemical redundancy of OcUGEs had not been documented. Hence, to obtain a comprehensive knowledge of OcUGEs, more extensive biochemical studies are performed in the present investigation.The effects of pH and temperature on OcUGEs activities were detected first. UDP-D-Xyl and UDP-D-Gal were used as substrates to assess the activities of OcUGE1 and OcUGE2 in different pHs (Fig. 7) and temperatures (Fig. 8). OcUGE1 exhibited UDP-glucose 4-epimerase activity in a broad range pH 4–11. The maximum UDP-glucose 4-epimerase activity of OcUGE1 was determined to be around pH 8.0. Moreover, OcUGE1 showed UDP-xylose 4-epimerase activity that was also maximal at about pH 8.0. The pH profiles of UDP-glucose 4-epimerase and UDP-xylose 4-epimerase activities of OcUGE2 were consistent with that of OcUGE1 (Fig. 7). The alkaline preference of OcUGEs activities was also occurred in UGEs isolated from other vascular plants.6,7
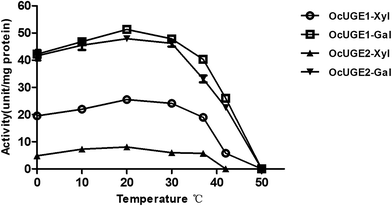
The optimum temperature for both activities of OcUGE1 was 20 °C, and the enzyme lost both activities completely at temperature higher than 50 °C (Fig. 8). Both optimum activities of OcUGE2 were also occurred at 20 °C (Fig. 8). In agreement with that of OcUGE1, the UDP-glucose 4-epimerase activity of OcUGE2 was also lost at temperature higher than 50 °C (Fig. 8). On the other hand, as long as the temperature exceeds 42 °C, the UDP-xylose 4-epimerase of OcUGE2 will be lost completely.
The effects of metal ions (Ca2+, Mg2+, Mn2+, NH4+) on the UDP-xylose 4-epimerase activity of OcUGE1 and OcUGE2 were also examined. Results showed the two purified recombinant enzymes did not require any exogenous supply of metal ion for their UDP-xylose 4-epimerase activity (Table 3). As expected from the results, the addition of 10 mM EDTA also has no effect on UDP-xylose 4-epimerase activity of OcUGE1 and OcUGE2 (Table 3). The metal ion independence of OcUGEs was shared by other UGEs from pea (Pisum sativum L.)6 and barley (Hordeum vulgare L.).7 On the contrary, several enzymes acting on nucleotide sugars require metal ions.47–51 For example, the sucrose breakdown activity of sucrose synthase was regulated by metal ions.27 Also, metal ions were found to influence the catalytic activities of UXS and UAXS from diverse plants.52,53 The discrepancy in metal ion dependence revealed the properties complexity of various nucleotide sugar interconversion enzymes. Also, the discrepancy showed we are far from understanding the nature of the bifunctional UGEs. Thus, the functional significance of metal ion dependence between UGEs and other nucleotide sugar interconversion enzymes remains to be established.
| Substrate (UDP-D-Xyl%) | Product (UDP-L-Ara%) | |
|---|---|---|
| OcUGE1 | ||
| Control | 46.4 | 53.6 |
| Ca2+ | 46.5 | 53.5 |
| Mg2+ | 46.8 | 53.2 |
| Mn2+ | 45.8 | 54.2 |
| NH4+ | 46.8 | 53.2 |
| EDTA | 46.5 | 53.5 |
| NAD+ | 46.9 | 53.1 |
| NADH | 45.7 | 54.3 |
| NADP+ | 46.3 | 53.7 |
| NADPH | 46.4 | 53.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| OcUGE2 | ||
| Control | 74.2 | 25.8 |
| Ca2+ | 73.9 | 26.1 |
| Mg2+ | 73.8 | 26.2 |
| Mn2+ | 74.1 | 25.9 |
| NH4+ | 74.5 | 25.5 |
| EDTA | 74.2 | 25.8 |
| NAD+ | 72.6 | 27.4 |
| NADH | 73.7 | 26.3 |
| NADP+ | 73.8 | 26.2 |
| NADPH | 74.5 | 25.5 |
Additionally, the effect of pyridine nucleotide co-enzymes (NAD+, NADH, NADP+, NADPH) on OcUGE activities was examined in the present study. As illustrated in Table 3, together with previous reports6,7 indicated addition of co-factors to the reaction mixture neither stimulated nor inhibited the UDP-xylose 4-epimerase activity of the recombinant OcUGE1 and OcUGE2. Actually, NAD+ was reported to play a critical role in the reaction mechanism of UGEs.54 It was therefore concluded that the recombinant OcUGE1 and OcUGE2 each contained one bound NAD+ molecule.7
Further, kinetic studies of OcUGE1 and OcUGE2 were performed for UDP-D-Glc, UDP-D-Gal, UDP-D-Xyl and UDP-L-Ara (Table 4). The resulting kinetic data for OcUGE1 and OcUGE2 are summarized in Table 4. OcUGE1 had an apparent Km of 412 (UDP-D-Xyl), 555 (UDP-L-Ara), 904 (UDP-D-Glc) and 661 μM (UDP-D-Gal), indicating a high affinity for UDP-D-Xyl. On the other hand, OcUGE2 showed a high affinity for UDP-D-Gal with a Km of 611 μM (Table 4). The Km values for OcUGE1 and OcUGE2 are comparable to those of UGE enzymes from pea (Pisum sativum L.),6 barley (Hordeum vulgare L.)7 and Arabidopsis thaliana.14 The turnover rate (kcat) values of OcUGE2 for UDP-D-Gal and UDP-D-Glc were 24.11 and 6.08, respectively. It is thus concluded that the efficiency of the reaction catalyzed by OcUGE2 was approx. 4-fold higher for UDP-D-Gal than for UDP-D-Glc, which is roughly in line with the observed equilibrium constant (Keq) between UDP-D-Gal and UDP-D-Glc in the reaction mixture containing OcUGE2 (Table 5). As illustrated in Table 5, when the reaction reached equilibrium, UDP-D-Gal and UDP-D-Glc concentrations were approx. 24 and 76% of total nucleotide sugars in the reaction system, respectively. The Keq obtained from the ratio of UDP-D-Gal to UDP-D-Glc was around 0.32. This result provided a clue that more UDP-D-Gal was transformed into UDP-D-Glc, indicating a higher efficiency of OcUGE2 for UDP-D-Gal than for UDP-D-Glc (Table 5). Likewise, OcUGE1 exhibited the higher activity in the conversion of UDP-D-Gal into UDP-D-Glc, whereas it showed a lower activity in the reverse direction (Table 5) with a ratio of approx. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between UDP-D-Glc and UDP-D-Gal at equilibrium (Table 3). Compared to a strong UGE activity, the UDP-xylose 4-epimerase activity inherent in OcUGE proteins is weak, which can be drawn from the kcat for UDP-D-Xyl and UDP-L-Ara and Keq between UDP-D-Xyl and UDP-L-Ara. In particular, the UXE activity of OcUGE2 is weaker than that of OcUGE1. OcUGE1 belongs to plant UGE I family together with the other counterparts, including AtUGE1 and AtUGE3 from Arabidopsis. The presence of a relatively high UXE activity in OcUGE1 support the notion that high UDP-xylose 4-epimerase activity is a common feature for the enzymes of the plant UGE I family.
1 between UDP-D-Glc and UDP-D-Gal at equilibrium (Table 3). Compared to a strong UGE activity, the UDP-xylose 4-epimerase activity inherent in OcUGE proteins is weak, which can be drawn from the kcat for UDP-D-Xyl and UDP-L-Ara and Keq between UDP-D-Xyl and UDP-L-Ara. In particular, the UXE activity of OcUGE2 is weaker than that of OcUGE1. OcUGE1 belongs to plant UGE I family together with the other counterparts, including AtUGE1 and AtUGE3 from Arabidopsis. The presence of a relatively high UXE activity in OcUGE1 support the notion that high UDP-xylose 4-epimerase activity is a common feature for the enzymes of the plant UGE I family.
| Substrate | Km (M) | Vmax (M s−1) | kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|---|
| OcUGE1 | ||||
| UDP-D-Xyl | 4.12 × 10−4 | 1.63 × 10−6 | 6.45 | 1.57 × 104 |
| UDP-L-Ara | 5.55 × 10−4 | 8.93 × 10−7 | 3.54 | 6.37 × 103 |
| UDP-D-Glc | 9.04 × 10−4 | 3.52 × 10−6 | 13.98 | 1.54 × 104 |
| UDP-D-Gal | 6.61 × 10−4 | 5.59 × 10−6 | 22.21 | 3.36 × 104 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| OcUGE2 | ||||
| UDP-D-Xyl | 9.88 × 10−4 | 6.92 × 10−7 | 2.69 | 2.72 × 103 |
| UDP-L-Ara | 9.35 × 10−4 | 3.89 × 10−7 | 2.52 | 2.69 × 103 |
| UDP-D-Glc | 9.57 × 10−4 | 1.56 × 10−6 | 6.08 | 6.35 × 103 |
| UDP-D-Gal | 6.11 × 10−4 | 6.12 × 10−6 | 24.11 | 3.95 × 104 |
| Substrate | Substrate (%) | Product (%) |
|---|---|---|
| OcUGE1 | ||
| UDP-D-Xyl | 47.9 | 52.1 |
| UDP-L-Ara | 52.4 | 47.6 |
| UDP-D-Glc | 75.9 | 24.1 |
| UDP-D-Gal | 26.5 | 73.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| OcUGE2 | ||
| UDP-D-Xyl | 73.6 | 26.4 |
| UDP-L-Ara | 30.5 | 69.5 |
| UDP-D-Glc | 75.4 | 24.6 |
| UDP-D-Gal | 23.7 | 76.3 |
3.6. Expression analysis of OcUGEs and OcUXEs in O. caudatum
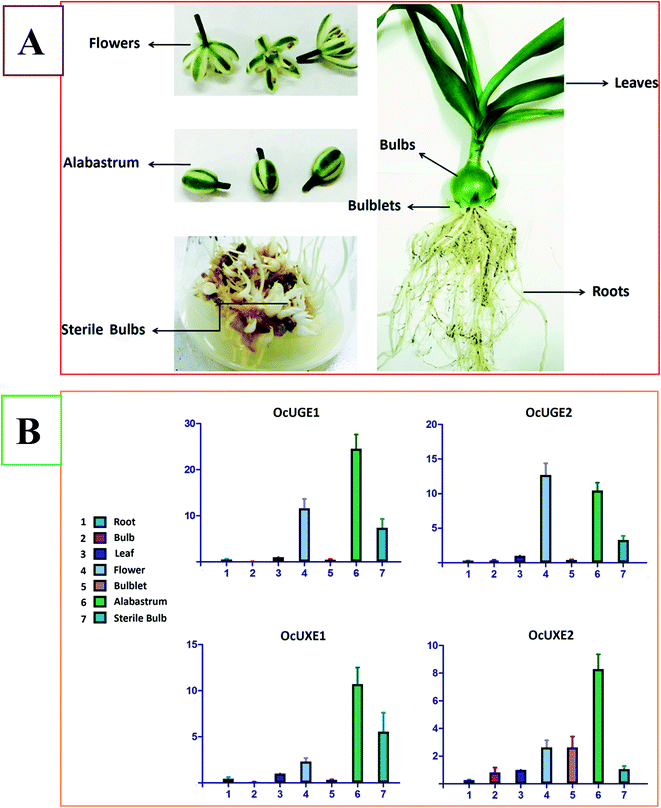
Galactose is the main component of OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3. It is well known that the incorporation of galactose into these polysaccharides requires the addition from UDP-D-Gal. In vascular plants, UGE is the enzyme responsible for the formation of UDP-D-Gal. UGE is therefore deemed to involve in the biosynthesis the four anticancer polysaccharides in O. caudatum. There are no exact evidences, however, to show which OcUGE genes are involved in the biosynthesis of galactose-containing polysaccharides. There are at least two UGE isoforms in O. caudatum genome. To verify the exact function of the two OcUGEs, it is essential to analyze their mRNA expression pattern in planta. RT-qPCR, which is growing in importance as a means to validate gene expression, is therefore performed to analyze the transcript levels of OcUGEs in various tissues including roots, leaves, bulbs, flowers, alabastrum, bulblets and sterile bulbs (Fig. 9A). Sterile bulbs are the O. caudatum bulbs cultivated in the sterile conditions (Fig. 9A). Previous activity screening assays indicated that polysaccharides crude from cultivated and sterile bulbs both have anticancer activity, suggesting the presence of a common operating pathway of galactose-containing polysaccharides in the two bulbs.27 This finding provides a clue that genes highly expressed in both the two bulbs are the exact members responsible for biosynthetic pathway of galactose-containing polysaccharides in O. caudatum.Fig. 9B illustrated the relative transcript level of the two OcUGE genes standardized to the constitutive GAPDH gene expression level. The expression of the two OcUGE genes was detected in all tissues tested, though at varied levels. This ubiquitous expression pattern of OcUGE genes is conserved in vascular plants. As indicated in Fig. 9B, OcUGE1 and OcUGE2 were expressed in bulbs, bulblets and sterile bulbs, but were predominant in sterile bulbs, suggesting both OcUGE1 and OcUGE2 are likely to be responsible for the biosynthesis of OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3. Sterile bulbs are cultivated in 6,7-V medium containing 80 g L−1 sucrose. The high levels of transcripts of OcUGE and OcUGE2 in sterile bulbs are, therefore, likely result from their sucrose-inducibility. The sucrose inducible property of OcUGEs is consistent with that of OcSus. In the previous study, we found high level of sucrose can induce high transcripts of OcSus in sterile bulbs of O. caudatum, thereby channeling more sucrose into the generation of UDP-D-Glc. Thus, more UDP-D-Gal was generated to form more galactose-containing polysaccharides as the activity of OcUGEs was elevated.
Since both UGE and UXE can catalyze the interconversion between UDP-D-Xyl and UDP-L-Ara, there are at least two pathways generating UDP-L-Ara in planta, namely the cytosol and Golgi pathway. UGEs freely interconvert UDP-D-Xyl and UDP-L-Ara in the cytosol, while membrane-anchored UXEs catalyze the same reaction in the Golgi apparatus. It concluded that UXEs also participated in the biosynthesis of arabinose-containing polysaccharides like OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3 in O. caudatum. As indicated as Fig. 9B, OcUXE1 highly expressed in sterile bulbs, suggesting its involvement in the biosynthesis of OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3. On the other hand, the expression levels of OcUXE2 gene in bulbs and sterile bulbs are almost the same, but are far lower than the expression level in the bulblet. The decreased transcript level of OcUGE2 in sterile bulbs revealed that OcUXE2 is not sucrose-inducible. Further, the same activity of OcUXE2 in bulbs and sterile bulb indicated the important role of OcUXE2 in bulb growth and development. Unlike the differential expression in the bulbs, bulblet and sterile bulbs, the four genes exhibited higher expression level in flower and alabastrum, suggesting that these gene also played predominant roles in carbohydrate metabolism during the flower development.
In conclusion, their involvement of OcUGE1, OcUGE2 and OcUXE1 in the biosynthesis of OCAP-2-1, OCAP-2-2, OCAP-3-1 and OCAP-3-3 was verified tentatively. However, to fully understand the function of these genes, there is still a lot of work to be performed.
4. Conclusions
UDP-D-Gal and UDP-L-Ara are two important activated sugar donors used for the biosynthesis of galactose- and arabinose-containing polysaccharides in O. caudatum. The cDNA isolation and functional significance of genes, including UGEs and UXEs, responsible for UDP-D-Gal and UDP-L-Ara biosynthesis had never been documented. Here, a full characterization of OcUGE and OcUXE gene families based on transcriptome-wide search was presented. One OcUGE gene family containing two members was functionally identified in vivo as a bifunctional 4-epimerase interconverting both UDP-D-Glc and UDP-D-Gal, and UDP-D-Xyl and UDP-L-Ara. Most important, OcUGE1, OcUGE2 and OcUXE1 were preliminarily revealed to be responsible for the biosynthesis of the four polysaccharides in O. caudatum.Abbreviations
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IPTG | Isopropyl β-D-thiogalactoside |
| ORF | Open reading frame |
| RT-qPCR | Real-time quantitative PCR |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| UAXS | UDP-D-apiose/UDP-D-xylose synthase |
| UDP-D-Gal | UDP-D-galactose |
| UDP-D-GalA | UDP-D-galacturonic acid |
| UDP-D-Glc | UDP-D-glucose |
| UDP-D-GlcA | UDP-D-glucuronic acid |
| UDP-D-Xyl | UDP-D-xylose |
| UGE | UDP-glucose 4-epimerases |
| UDP-L-Ara | UDP-L-arabinose |
| UDP-GalNAc | UDP-N-acetylgalactosamine |
| UDP-GlcNAc | UDP-N-acetylglucosamine |
| UglcNAcE | UDP-N-acetylglucosamine 4-epimerase |
| UXE | UDP-xylose 4-epimerase |
| UXS | UDP-xylose synthase |
Acknowledgements
The authors thank Profs. Cheng KD and Wang W for providing the sterile bulb of Ornithogalum caudatum. This work was supported by Independent Subject of Key Project of State Key Laboratory of Bioactive Substance and Function of Natural Medicines (GTZA201404) and Institute of Materia Medica Foundation (2016ZD01).References
- Y. Yin, J. Huang, X. Gu, M. Bar-Peled and Y. Xu, PLoS One, 2011, 6, e27995 CAS.
- M. Bar-Peled and M. A. O'Neill, Annu. Rev. Plant Biol., 2011, 62, 127–155 CrossRef CAS PubMed.
- W. D. Reiter, Curr. Opin. Plant Biol., 2008, 11, 236–243 CrossRef CAS PubMed.
- G. J. Seifert, Curr. Opin. Plant Biol., 2004, 7, 277–284 CrossRef CAS PubMed.
- W. D. Reiter and G. F. Vanzin, Plant Mol. Biol., 2001, 47, 95–113 CrossRef CAS PubMed.
- T. Kotake, R. Takata, R. Verma, M. Takaba, D. Yamaguchi, T. Orita, S. Kaneko, K. Matsuoka, T. Koyama, W. D. Reiter and Y. Tsumuraya, Biochem. J., 2009, 424, 169–177 CrossRef CAS PubMed.
- Q. Zhang, M. Hrmova, N. J. Shirley, J. Lahnstein and G. B. Fincher, Biochem. J., 2006, 394, 115–124 CrossRef CAS PubMed.
- P. Dormann and C. Benning, Plant J., 1998, 13, 641–652 CrossRef CAS PubMed.
- S. Wang, T. Ito, M. Uehara, S. Naito and J. Takano, J. Plant Res., 2015, 128, 863–873 CrossRef CAS PubMed.
- D. R. Guevara, A. El-Kereamy, M. W. Yaish, Y. Mei-Bi and S. J. Rothstein, PLoS One, 2014, 9, e96158 Search PubMed.
- M. J. Wubben II, S. R. Rodermel and T. J. Baum, Plant J., 2004, 40, 712–724 CrossRef PubMed.
- J. Rosti, C. J. Barton, S. Albrecht, P. Dupree, M. Pauly, K. Findlay, K. Roberts and G. J. Seifert, Plant Cell, 2007, 19, 1565–1579 CrossRef CAS PubMed.
- E. G. Burget, R. Verma, M. Molhoj and W. D. Reiter, Plant Cell, 2003, 15, 523–531 CrossRef CAS.
- C. Barber, J. Rosti, A. Rawat, K. Findlay, K. Roberts and G. J. Seifert, J. Biol. Chem., 2006, 281, 17276–17285 CrossRef CAS PubMed.
- Q. Zhang, N. J. Shirley, R. A. Burton, J. Lahnstein, M. Hrmova and G. B. Fincher, Plant Physiol., 2010, 153, 555–568 CrossRef CAS PubMed.
- A. L. Pey, E. Padin-Gonzalez, N. Mesa-Torres and D. J. Timson, Arch. Biochem. Biophys., 2014, 562, 103–114 CrossRef CAS PubMed.
- S. Suzuki, T. Matsuzawa, Y. Nukigi, K. Takegawa and N. Tanaka, Microbiology, 2010, 156, 708–718 CrossRef CAS PubMed.
- A. Brahma and D. Bhattacharyya, FEBS Lett., 2004, 577, 27–34 CrossRef CAS PubMed.
- J. B. Thoden and H. M. Holden, Biochemistry, 1998, 37, 11469–11477 CrossRef CAS PubMed.
- X. Gu, S. G. Lee and M. Bar-Peled, Microbiology, 2011, 157, 260–269 CrossRef CAS PubMed.
- P. Dörmann and C. Benning, Arch. Biochem. Biophys., 1996, 327, 27–34 CrossRef PubMed.
- M. R. Lake, C. L. Williamson and R. D. Slocum, Plant Physiol. Biochem., 1998, 36, 555–562 CrossRef CAS.
- D.-F. Fan and D. S. Feingold, Plant Physiol., 1969, 44, 599–604 CrossRef CAS PubMed.
- D. F. Fan and D. S. Feingold, Plant Physiol., 1970, 46, 592–595 CrossRef CAS PubMed.
- Y. Tang, B. Yu, J. Hu, T. Wu and H. Hui, J. Nat. Prod., 2002, 65, 218–220 CrossRef CAS.
- R. Chen, F. Meng, Z. Liu, R. Chen and M. Zhang, Carbohydr. Polym., 2010, 80, 845–851 CrossRef CAS.
- L.-N. Li and J. Q. Kong, RSC Adv., 2016, 6, 18778–18792 RSC.
- L. Guo, X. Chen, L.-N. Li, W. Tang, Y.-T. Pan and J.-Q. Kong, Microb. Cell Fact., 2016, 15, 27 CrossRef PubMed.
- I. A. Veliky and S. M. Martin, Can. J. Microbiol., 1970, 16, 223–226 CrossRef CAS PubMed.
- Z.-B. Wang, X. Chen, W. Wang, K.-D. Cheng and J.-Q. Kong, RSC Adv., 2014, 4, 27159–27175 RSC.
- J. Q. Kong, D. Lu and Z. B. Wang, Molecules, 2014, 19, 1608–1621 CrossRef PubMed.
- L. Guo and J.-Q. Kong, Z. Naturforsch., C: J. Biosci., 2014, 69, 259–270 CAS.
- M. G. Grabherr, B. J. Haas, M. Yassour, J. Z. Levin, D. A. Thompson, I. Amit, X. Adiconis, L. Fan, R. Raychowdhury, Q. Zeng, Z. Chen, E. Mauceli, N. Hacohen, A. Gnirke, N. Rhind, F. di Palma, B. W. Birren, C. Nusbaum, K. Lindblad-Toh, N. Friedman and A. Regev, Nat. Biotechnol., 2011, 29, 644–652 CrossRef CAS PubMed.
- Z. Wang, M. Gerstein and M. Snyder, Nat. Rev. Genet., 2009, 10, 57–63 CrossRef CAS PubMed.
- M. Joersbo, S. G. Pedersen, J. E. Nielsen, J. Marcussen and J. Brunstedt, Plant Sci., 1999, 142, 147–154 CrossRef CAS.
- G. Kleiger and D. Eisenberg, J. Mol. Biol., 2002, 323, 69–76 CrossRef CAS PubMed.
- O. Dym and D. Eisenberg, Protein Sci., 2001, 10, 1712–1728 CrossRef CAS PubMed.
- N. Tanaka, T. Nonaka, K. T. Nakamura and A. Hara, Curr. Org. Chem., 2001, 5, 89–111 CrossRef CAS.
- K. Fujimoto, M. Hara, H. Yamada, M. Sakurai, A. Inaba, A. Tomomura and S. Katoh, Chem.-Biol. Interact., 2001, 130, 825–832 CrossRef PubMed.
- S. Y. Kim, H. R. Lee, K. S. Park, B. G. Kim and J. H. Ahn, Appl. Microbiol. Biotechnol., 2015, 99, 2233–2242 CrossRef CAS PubMed.
- B. G. Kim, S. M. Yang, S. Y. Kim, M. N. Cha and J. H. Ahn, Appl. Microbiol. Biotechnol., 2015, 99, 2979–2988 CrossRef CAS PubMed.
- S. H. Han, B. G. Kim, J. A. Yoon, Y. Chong and J. H. Ahn, Appl. Environ. Microbiol., 2014, 80, 2754–2762 CrossRef CAS PubMed.
- M. Y. Victor and S. K. Bhatia, Biotechnol. Lett., 2012, 34, 1405–1414 CrossRef PubMed.
- S. M. Ma, D. E. Garcia, A. M. Redding-Johanson, G. D. Friedland, R. Chan, T. S. Batth, J. R. Haliburton, D. Chivian, J. D. Keasling and C. J. Petzold, Metab. Eng., 2011, 13, 588–597 CrossRef CAS PubMed.
- F. Baneyx and M. Mujacic, Nat. Biotechnol., 2004, 22, 1399–1408 CrossRef CAS PubMed.
- K. Nishihara, M. Kanemori, M. Kitagawa, H. Yanagi and T. Yura, Appl. Environ. Microbiol., 1998, 64, 1694–1699 CAS.
- S. Pattathil, A. D. Harper and M. Bar-Peled, Planta, 2005, 221, 538–548 CrossRef CAS PubMed.
- M. Diricks, F. De Bruyn, P. Van Daele, M. Walmagh and T. Desmet, Appl. Microbiol. Biotechnol., 2015, 99, 8465–8474 CrossRef CAS PubMed.
- U. Romer, H. Schrader, N. Gunther, N. Nettelstroth, W. B. Frommer and L. Elling, J. Biotechnol., 2004, 107, 135–149 CrossRef CAS PubMed.
- K. Tanase and S. Yamaki, Plant Cell Physiol., 2000, 41, 408–414 CrossRef CAS PubMed.
- L. Elling, M. Grothus and M. R. Kula, Glycobiology, 1993, 3, 349–355 CrossRef CAS PubMed.
- A. D. Harper and M. Bar-Peled, Plant Physiol., 2002, 130, 2188–2198 CrossRef CAS PubMed.
- M. Molhoj, R. Verma and W. D. Reiter, Plant J., 2003, 35, 693–703 CrossRef CAS PubMed.
- A. J. Bauer, I. Rayment, P. A. Frey and H. M. Holden, Proteins, 1992, 12, 372–381 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03817d |
| This journal is © The Royal Society of Chemistry 2016 |