Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of strength and size of donors on the optoelectronic properties of D–π–A sensitizers†
J.
Sivanadanam
a,
P.
Ganesan
b,
Peng
Gao
 *b,
Md. K.
Nazeeruddin
*b,
Alexei
Emeline
cd,
Detlef
Bahnemann
cd and
R.
Rajalingam
*a
*b,
Md. K.
Nazeeruddin
*b,
Alexei
Emeline
cd,
Detlef
Bahnemann
cd and
R.
Rajalingam
*a
aSchool of Chemistry, Bharathidasan University, Tiruchirappalli 620024, Tamilnadu, India. E-mail: rrengas@gmail.com
bGroup for Molecular Engineering of Functional Materials, EPFL Valais Wallis, Rue de l’industrie 17, CH-1951 Sion, Switzerland. E-mail: peng.gao@epfl.ch; mdkhaja.nazeeruddin@epfl.ch
cLaboratory “Photoactive Nanocomposite Materials”, Saint-Petersburg State University, Ulyanovskaya str. 1 Peterhof, Saint-Petersburg, 198504 Russia. E-mail: detlef.bahnemann@spbu.ru
d“Photocatalysis and Nanotechnology”, Institut fuer Technische Chemie, Gottfried Wilhelm Leibniz Universitaet Hannover, Callinstrasse 3, D-30167 Hannover, Germany. E-mail: bahnemann@iftc.uni-hannover.de
First published on 15th April 2016
Abstract
A series of carbazole based sensitizers with either phenyl based donors (TBC, TMC, OMC, PC, TBR, TMR, OMR and PR) or aryl amine based donors (OMNC, CNC and HNC) as well as one without a donor group (CC) have been synthesized to understand the influence of the strength of the donor moiety on the optical, electrochemical and photovoltaic properties. Two different acceptor moieties such as cyano acrylic acid and rhodanine acetic acid were introduced and evaluated. Different substituents on the phenyl group have a significant impact on the light harvesting ability of the sensitizers. Among phenyl based donors, anisole based carbazole (OMC) shows the highest short circuit current (JSC) of 4.96 mA cm−2 with overall power conversion efficiency (PCE) of 2.69%. In the case of the sensitizers with aryl amine based donors, the increasing bulkiness of the donor group lead to increasing open circuit potential. Transient photocurrent and photovoltage measurements signify the importance of a bulky donor fragment in determining the open circuit potential of the dyes. Sensitizers with hexyloxy substituted phenyl amine as the donor group shows a JSC of 6.84 mA cm−2 with PCE of 3.33%. The overall investigation provides vital information about the influence of donor groups on the optoelectronic properties of the sensitizers for its photovoltaic applications.
1. Introduction
Dye sensitized solar cells (DSSC) are one of the most promising methods to convert solar energy to electrical energy at low cost with high power conversion efficiency.1,2 DSSC comprises four major components i.e., a semiconductor metal oxide, sensitizer, redox active electrolyte and counter electrode. Sensitizers play a major role in harvesting visible light radiations to achieve high power conversion efficiency and have been intensively studied. To date, dyes based on ruthenium complexes and porphyrin sensitizers achieved maximum power conversion efficiencies of 11 and 13%, respectively.1,3 Development of metal free organic sensitizers has been increased vastly in this decade due to the rare, expensive ruthenium metal and the difficulties in purification process. Organic sensitizers however have advantages like high molar extinction coefficient, structural flexibility, low cost, easier preparation and purification process. In the past years, sensitizers with various aromatic building block such as coumarin,4 indoline,5 perylene,6 merocyanine,7 porphyrin,8 triarylamine9 and carbazole10 have been reported, all of which have obtained power conversion efficiency in the range of 5–9%.Carbazole is a well-known heterocyclic compound which has unique optoelectronic properties, and has found applications in hole transporting material in organic devices, electron donors in solar cells and host material in electroluminescent devices.11,12 Chemically, carbazole is easily functionalized at 3, 6 and 9 positions and covalently bonded to other molecular compounds.13,14 Recently, Jianhua Su14 reported the D–π–A molecules functionalized at the above positions showing the efficiency up to 5.91%. Most recently, sensitizer (MK-2) based on carbazole reported by Z. S. Wang and co-workers produced a maximum power conversion efficiency of 8.3%.13 The classic structural arrangement for organic dyes comprises the donor fragment and acceptor fragment linked by the π spacer (D–π–A). Among various types of donor groups utilized in the DSSC, aryl amine based donor groups have been most popular options achieving reasonable efficiencies.15 Increasing the twist angle between the donor and π spacer will avoid the dye aggregation that eventually assists in reducing the charge recombination between the injected electrons and the oxidized dyes. Recently, fluorenes and dibenzo heterocycles such as carbazole were successfully employed as π spacer by gratifying the above mentioned criteria.16 Further functionalization of different donor moieties in the carbazole fragment is easy and convenient that will aid in constructing the required donor–π–acceptor structural arrangement. In this context, a complete study on the impact of strength of donor groups in a simple D–π–A sensitizers will unveil the role of the donor group in the light harvesting ability, electrochemical and photovoltaic parameters.
Based on these points, in this paper we have synthesised carbazole based sensitizers with/without donor groups of different donating strength. All the new sensitizers were successfully synthesised and characterized. The differences in the strength of the donor fragments reflected in the absorption, emission, electrochemical properties of the carbazole sensitizers. The variations in the photovoltaic parameters such as short circuit current density JSC, open circuit potential VOC by varying the substituents in the donor fragments were discussed. The potential of the sensitizers to convert the absorbed light in to current was analysed using IPCE measurements. Transient photocurrent and photovoltage measurements were carried out to analyse the importance of bulky donor fragment in determining the open circuit potential of the dyes.
2. Experimental part
Carbazole, 1-bromo octane, rhodanine acetic acid, (2,4,6-trimethylphenyl)boronic acid, 4-methoxyphenylboronic acid, 4-phenylboronic acid and Pd(PPh3)4 were purchased from Aldrich. 1,2-Dichloroethane, sodium hydroxide, potassium iodide, potassium carbonate and 100–200 mesh silica gel were from Merck chemicals. Phosphorous oxy chloride, cyano acetic acid and ammonium acetate were obtained from Loba chemicals. 4-tert-Butylphenylboronic acid and potassium iodate from Spectrochem. All solvents were purchased from spectrometric grade and used as received without further purification. 1H NMR and 13C NMR spectrum was recorded on a Bruker 400 MHz spectrometer in CDCl3-d or DMSO-d6 solvent with tetramethylsilane as internal standard. Mass spectra were obtained with Bruker daltonics, FT-ICR/APEX II, ESI mode. MALDI-TOF mass spectra were recorded using Micromass Tof Spec 2E instrument. High-resolution mass spectra were obtained at the École Polytechnique Fédérale de Lausanne mass spectrometry laboratory (EPFL).Absorption spectra of dyes in chloroform solution and on TiO2 films were recorded using a JASCO 300 UV-Visible spectrometer. Fluorescence measurements were carried out using Perkin Elmer LS 55 spectrofluorimeter with 10 nm slit width and 200 nm min−1 scan width was kept constant for all measurements. Electrochemical studies were examined by cyclic voltammetry (SP-50 model potentiostat) using a three electrode system. The working electrode was a platinum electrode, the counter electrode was a platinum wire and the reference electrode was a Ag/AgCl. Tetrabutylammonium hexafluoro phosphate (TBAPF6, 0.1 M) was used as supporting electrolyte in acetonitrile solution for phenyl based donors and in dichloromethane solutions for amine based donors. A pinch of ferrocene was added to each sample and ferrocene/ferrocenium (Fc/Fc+) was used as the internal standard. The potential of the dyes vs. normal hydrogen electrode (NHE) were calibrated by using ferrocene (0.630 V vs. NHE) as the internal standard.17 All calculations were carried out using Spartan'10 Windows run on Microsoft Windows XP, Geometry optimization, energy levels, and frontier molecular orbitals of the dyes' HOMOs and LUMOs were calculated at the B3LYP/6-31G (d,p) level.
A 450 W xenon lamp (Oriel, USA) was used as a light source to study the current–voltage characteristics of the DSSC. The spectral output of the lamp was filtered using a Schott K113 Tempax sunlight filter (Präzisions Glas & Optik GmbH, Germany) to reduce the mismatch between the simulated and actual solar spectrum to less than 2%. The Keithley model 2400 digital source meter (Keithley, USA) was used for data acquisition. The photo-active area of 0.16 cm2 was defined by a black mask of 6 × 6 mm2. Incident photon-to-current conversion efficiency measurements were measured using the mono chromated visible photons, from Gemini-180 double monochromator Jobin Yvon Ltd (UK), powered by a 300 W xenon light source (ILC Technology, USA) superimposed on a 10 mW cm−2 LED light. The monochromatic incident light was passed through a chopper running at 2 Hz frequency and the on/off ratio was measured by an operational amplifier. Photovoltage transients were observed by using a pump pulse generated by 4 red light emitting diodes controlled by a fast solid-state switch with a white light bias. The pulse of red light with widths of 50 ms was incident on the photoanode side of the cell, and its intensity was controlled to keep a suitably low level to generate the exponential voltage decay where the charge recombination rate constants are obtained directly from the exponential decay rate.18
3. Device fabrication
A screen-printed double layer of nanocrystalline TiO2 particles was used as the photoelectrode. The FTO glass plates were immersed in a 40 mM aqueous TiCl4 solution at 70 °C for 30 min and washed with water and ethanol. A 8 μm thick film of 20 nm sized TiO2 particles was then printed on the FTO conducting glass and further coated with a 5 μm thick second layer of light-scattering TiO2 particles (400 nm diameter, Catalysts & Chemicals Ind. Co. Ltd (CCIC), HPW-400). Sintering was carried out at 500 °C for 15 min, which was gradually heated. The working electrode was prepared by immersing the 13.0 μm (8.0 μm thick transparent layer + 5.0 μm thick scattering) TiO2 film into the dye solution for 12 h. To prepare the counter electrode, Pt catalyst was deposited on cleaned FTO glass by coating with a drop of H2PtCl6 solution (10 mM in 2-propanol solution) with heat treatment at 400 °C for 15 min. For the assembly of DSSCs, the dye-containing TiO2 electrode and Pt counter electrode were assembled into a sandwich-type cell and sealed with a hot-melt gasket of 25 microns thickness made of the ionomer Surlyn 1702 (Dupont). Devices were completed by filling the electrolyte through the pre-drilled holes in the counter electrodes and finally the holes were sealed with a Surlyn sheet and a thin glass cover by heating. A black mask (6 × 6 mm) was used in the subsequent photovoltaic studies.4. Synthesis and characterization of carbazole based sensitizers
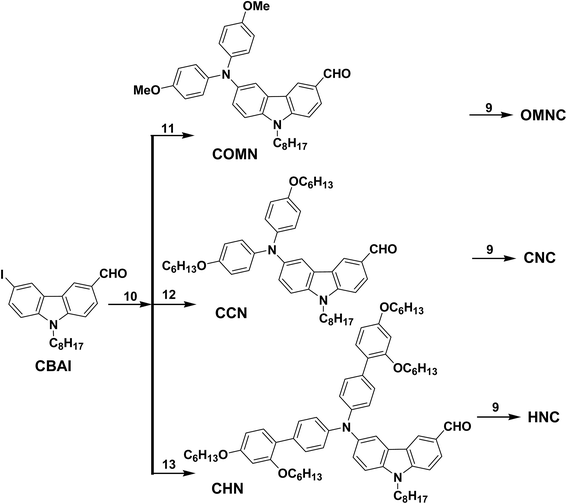
The complete synthetic procedures of all intermediate compounds are available in ESI.† Three amine donor moieties (11, 12 and 13) are synthesized according to the literature.19,204.1. General procedure for the preparation of target compounds
To a mixture of corresponding aldehydes (1 mol ratio), cyanoacetic acid or rhodanine acetic acid (1.5 mol ratio) and ammonium acetate (1.95 mol ratio) in glacial acetic acid (5 ml) was stirred at 80 °C for 1 day. After completion of the reaction, cool to RT followed by quench the mixture in ice water, a precipitate was obtained. It was filtered and dried to afford the desired product.5. Results and discussion
5.1. Synthesis and characterization
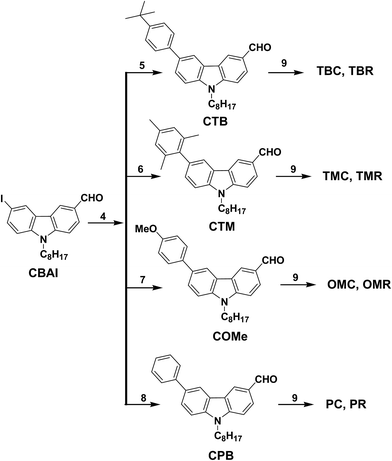
Schemes 1 and 2 shows the structures of carbazole based sensitizers having donor fragments with different strength. The synthetic route of CBAI is shown in Scheme 3. CB is prepared from carbazole via N-alkylation with 1-bromo octane, followed by Vilsmeier hack formylation reaction to get CBA. Iodination of CBA using KI and KIO3 afforded CBAI. Four different types of aryl boronic acid (4-tert-butylphenylboronic acid, (2,4,6-trimethylphenyl)boronic acid, 4-methoxyphenylboronic acid and 4-phenylboronic acid) were coupled with CBAI via Suzuki coupling reaction to get their corresponding aldehydes. A peak around 10.09–10.1 ppm in proton NMR clearly shows the presence of the aldehyde group. Knoevenagel condensation of aldehydes precursors with cyano acetic acid or rhodanine acetic acid attains the target compounds as shown in Scheme 4. CBAI undergoes Buchwald coupling with three different types of aryl amines to afford the respective aldehydes followed by Knoevenagel condensation to obtain final compounds as depicted in Scheme 5. Similarly CBA (absence of donating group) undergo Knoevenagel condensation with cyano acetic acid in the presence of ammonium acetate and acetic acid to give CC. These new molecules are characterized by 1H, 13C NMR, melting point measurement and mass spectroscopy techniques. | ||
| Scheme 3 Synthetic pathways for the preparation of CBAI. Reagents: (1) carbazole, 1-bromo octane, NaOH, DMSO, RT. (2) CB, DMF, POCl3, DCE, 90 °C. (3) CBA, KI, KIO3, acetic acid, 80 °C. | ||
5.2. Optical properties
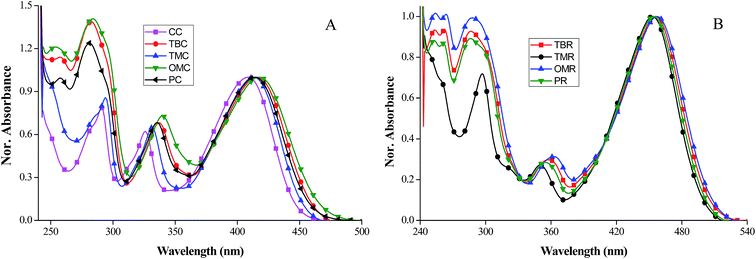
Fig. 1A and B shows the absorption spectra of phenyl donor substituted sensitizers in chloroform solvent and the data are summarized in Table 1. All the sensitizers shows two types of absorption bands, one at higher energy around 250–360 nm, which arise from π–π* transition and another band at lower energy appears at 360–540 nm which can be assigned to intramolecular charge transfer transition from electron donating moiety (CTB, CTM, COMe, CPB) to electron acceptor moiety (CAA, RA).21 Due to the difference in acceptor groups (CAA or RA), the intramolecular charge transfer bands occurred in different wavelength, e.g. 408 to 418 nm for CAA acceptor groups and 453 to 457 nm for RA acceptor groups (Fig. S1†). While analyzing the effect of various substituents in the phenyl donor on carbazole unit, we didn't observe any major difference in the absorption spectrum, only slight shifts are observed. By varying the donor moiety on carbazole unit the higher energy band is red shifting in the order of CC (408 nm) < TMC (413 nm) < PC (414 nm) < TBC (416 nm) < OMC (418 nm) for cyano acrylic acid substituted carbazole sensitizers and when rhodanine is used as acceptor, an order of TMR (453 nm) < PR (454 nm) < TBR (456 nm) < OMR (457 nm) is observed. Among all compounds, methoxyphenyl incorporated sensitizer (OMC) shows the most red-shifted absorption wavelength. TBR with tert-butyl phenyl as donating moiety shows bathochromic absorption shift by 3 nm compared to TMC. Due to the introduction of methoxy groups in para position of phenyl ring, OMC (λmax = 418 nm, ε = 7.66 × 105 M−1 cm−1) shows three times higher molar extinction coefficient than PC (λmax = 414 nm, ε = 4.36 × 105 M−1 cm−1) The molar extinction coefficient of TBC (λmax = 416 nm, ε = 2.66 × 105 M−1 cm−1) are slightly higher than TMC (λmax = 413 nm, ε = 1.97 × 105 M−1 cm−1) and CC (λmax = 408 nm, ε = 1.70 × 105 M−1 cm−1). RA derivatives shows molar extinction coefficient in the range of (1.66 to 3.23 × 105 M−1 cm−1). It is worth noting that inspite of simple structures of these sensitizers, their molar extinction coefficient are significantly higher than those of ruthenium sensitizers.22| λ max a (nm)/[ε × 105 (M−1 cm−1)] | λ max a (nm) on TiO2 film | λ emi a (nm) | E ox b (V) vs. NHE | E 0–0 c (eV) vs. NHE | E ox–E0–0 (V) | |
|---|---|---|---|---|---|---|
| a Absorption and emission spectrum of phenyl donor based carbazole dyes were measured in CHCl3 solutions. b Cyclic voltammograms of the first oxidation potential of the dyes were measured in acetonitrile solution containing 0.1 M TBAPF6 used as an supporting electrolyte with a scan rate of 100 mV s−1 (working electrode: Pt, counter electrode: Pt wire, reference electrode: Ag/AgCl calibrated with ferrocene/ferrocenium (Fc/Fc+) as an internal reference and converted to NHE by adding 630 mV). c The E0–0 transition values was estimated from the onset of absorption and emission spectra. | ||||||
| TBC | 416 (2.66) | 399 | 530 | 1.15 | 2.71 | −1.56 |
| TMC | 413 (1.97) | 371 | 500 | 1.58 | 2.79 | −1.21 |
| OMC | 418 (7.66) | 404 | 545 | 1.39 | 2.66 | −1.27 |
| PC | 414 (4.36) | 400 | 518 | 1.54 | 2.74 | −1.19 |
| CC | 408 (1.70) | 403 | 480 | 1.53 | 2.85 | −1.32 |
| TBR | 456 (1.66) | 371 | 537 | 1.44 | 2.52 | −1.08 |
| TMR | 453 (2.74) | 371 | 523 | 1.44 | 2.56 | −1.12 |
| OMR | 457 (3.23) | 371 | 553 | 1.30 | 2.49 | −1.19 |
| PR | 454/1.86 | 371 | 530 | 1.45 | 2.54 | −1.09 |
The absorption behavior of the sensitizers attached to the TiO2 surface is depicted in Fig. 2. Those absorption spectra are broadened and blue shifted as compared in solution state due to the strong interaction between dyes and semiconductor surface. Hypsochromic shift of 5 nm to 42 nm for cyano acrylic acid based compounds (OMC, PC, TBC, CC and TMC) occurred due to the formation of H type aggregation or deprotonation of the dyes on the surface of the TiO2 films.17,23 Rhodanine acetic acid based dyes (TBR, TMR, OMR and PR) shows more prominent blue shift by ∼86 nm owing to the strong deprotonation of carboxylic acid onto the TiO2 surface (Fig. S2†). While comparing these two acceptor moieties, rhodanine acetic acid substituted compounds absorb at longer wavelength than that of cyano acrylic acid based compounds both in chloroform solution and TiO2 film.
 | ||
| Fig. 2 Normalised absorption spectrum of TBC, TMC, OMC, PC and CC dyes in CHCl3 solution and on TiO2 films. | ||
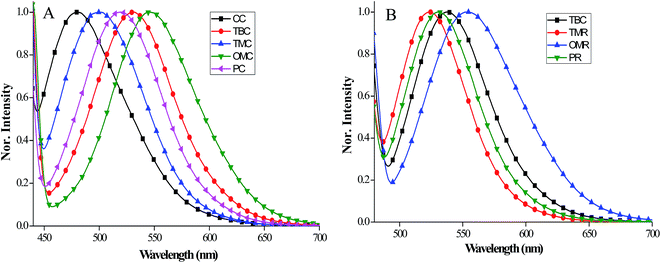
To further increase the donor strength, we choose phenyl amine based donor groups. Aryl amine donors with different alkyl chain substitution were synthesized to afford OMNC, CNC and HNC. Fig. 3 shows the absorption spectra of OMNC, CNC and HNC and the data is displayed in Table 2. Firstly, OMNC with para-substituted methoxy groups on diphenylamine shows the maximum absorption at 382 and 455 nm. By increasing the alkyl chain from methoxy to hexyloxy, a slightly red shifted absorption is observed at 382 and 459 nm. However, by introducing one more phenyl group with two hexyloxy groups attached in ortho and para positions leads to blue shifted absorption at 331 nm, which indicates the decreased donating strength of amine donor. Replacing phenyl donors with stronger amine donor results in red shifted onset absorption values which appear around 570 nm. Among sensitizers with phenyl donors the onset values appeared around 490 nm for CAA anchoring group and 530 nm for RA anchoring unit. The maximum absorption coefficient of the amine donor based sensitizers are in the order of HNC (ε = 3.93 × 104 M−1 cm−1 at 387 nm) > CNC (ε = 5.94 × 103 M−1 cm−1 at 459 nm) > OMNC (ε = 1.56 × 103 M−1 cm−1 at 455 nm). Increasing bulkiness via alkylation in the donor fragment prevents the dye aggregation which increases their molar absorption coefficient. The emission spectra of all carbazole sensitizers are recorded in chloroform and displayed in Fig. 4A and B. The emission data are collected in Table 1 and the values of different donor compounds are appeared in the same order of absorption maximum. Fluorescence spectra of amine donor dyes are displayed in Fig. 5 and the data is given in Table 2. When we look over the absorption spectra, a similar maximum absorption peak is observed for OMNC and CNC. However, the maximum emission peak of CNC is observed at 618 nm which is 125 nm higher than OMNC (493 nm). This reveals that the presence of alkyl chains in the donor fragment have significant impact in changing the excited state of the molecule.
| λ max a (nm)/[ε (M−1 cm−1)] | λ emi a (nm) | E ox b (V) vs. NHE | E 0–0 c (eV) vs. NHE | E ox–E0–0 (V) | |
|---|---|---|---|---|---|
| a Absorption and emission spectrum of amine donor based carbazole dyes were measured in CHCl3 solutions. b Cyclic voltammograms of the first oxidation potential of the dyes were measured in dichloromethane solution containing 0.1 M TBAPF6 used as an supporting electrolyte (working electrode: Pt, counter electrode: Pt wire, reference electrode: Ag/AgCl calibrated with ferrocene/ferrocenium (Fc/Fc+) as an internal reference and converted to NHE by adding 630 mV). c The E0–0 transition values was estimated from the onset of absorption and emission spectra. | |||||
| OMNC | 382 (4560) 455 (1560) | 493 | 0.73 | 2.81 | −2.08 |
| CNC | 382 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 459 (5940) 900) 459 (5940) |
618 | 1.18 | 2.92 | −1.74 |
| HNC | 331 (67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 387 (39 200) 387 (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
513 | 0.83 | 2.75 | −1.92 |
5.3. Electrochemical properties
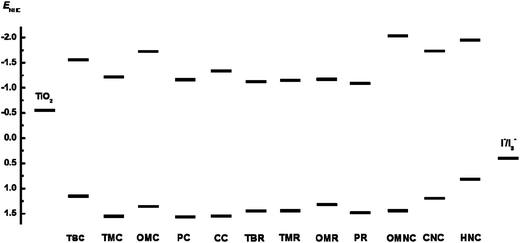
The first oxidation potential of the carbazole based sensitizers corresponds to the ground state potential level (i.e. HOMO) of the dye and is important for their photovoltaic applications. Cyclic voltammetry measurements were carried out to analyze the HOMO level of the dyes using tetrabutyl ammonium hexafluoro phosphate (TBAPF6) as the supporting electrolyte. Ferrocene/ferrocenium (Fc/Fc+) is used as an internal standard. The electrochemical data of all carbazole sensitizers are presented in Fig. 6 and summarized in Table 1. Single oxidation peaks are observed for CC, PC, PR and TMR and two oxidation peaks are observed for OMC, OMR, TBR and TMC and three oxidation peaks is observed for TBC. The first oxidation peaks of all dyes and second oxidation peak of OMC, OMR and TMC are reversible in nature, which suggest the oxidized states of the dyes are quite stable.24PC, TBR and TMR display quasi-reversible second oxidation peaks. The first oxidation peaks of all dyes occurs in the range of 1.15–1.58 V, which corresponds to the removal of electron from the donor moiety and second oxidation peaks occur at higher potentials, which can be attributed to the removal of electron from conjugated backbone of the dyes.25 The oxidation potential of the sensitizers with amine donor in dichloromethane solutions are depicted in Fig. 7 and Table 2. The first oxidation potentials of OMNC, CNC and HNC are 0.73 V, 1.18 V and 0.83 V, respectively. All the oxidation peaks are reversible in nature, showing that the oxidized states of these dyes are quite stable. The HOMO of the amine donor dyes occurs at lower potential than the phenyl donor dyes which is due to the strong donating ability of amine donor which results in easy removal of electrons and oxidise at lower potential. The HOMO level of the dyes are more positive than I−/I3− electrolyte (0.4 V vs. NHE), which reveals the efficient dye regeneration from electrolytes.The excited state oxidation potential (i.e. LUMO) can be calculated by subtracting zero–zero excitation energy (E0–0) from HOMO level of the dyes. The E0–0 energy values can be obtained from the point of intersection of the experimental absorption and emission spectra of sensitizers and the data is displayed in Table 1. The obtained LUMO values are sufficient for electron injection into conduction band potential of TiO2 (−0.5 V vs. NHE). The electrochemical data supports the thermodynamic driving force for efficient dye regeneration and electron injection in DSSC (Fig. 8).
The geometries of the dyes are optimized by density functional theory (DFT) calculations at the B3LYP.6-31G(d)21 level to analyze further the distribution of HOMO and lowest unoccupied molecular orbital (LUMO) energy levels of the sensitizers. Fig. S3A–F and Table S1† display the electron distributions of the HOMO and LUMO of the dyes. HOMOs are mainly delocalized in the phenyl and carbazole groups in the phenyl based donor fragment, while it resides mainly on the amino substituents in the amine based donor group. LUMOs show localized electron distributions on the acceptor parts cyanoacrylic acid and rhodanine acetic acid. The observed spatially separated frontier orbitals strongly promote intramolecular charge separation and hence favor efficient electron injection from the excited state of the dye into the semiconducting oxide.
5.4. Photovoltaic properties
| J SC (mA cm−2) | V OC (V) | ff | η (%) | |
|---|---|---|---|---|
| a TiO2 film has a 8 μm scattering layer and a 5 μm transparent layer. Electrolyte composition: 1.0 M DMII (1,3-dimethylimidazolium iodide), 0.03 M iodine, 0.025 M NaI, 0.5 M TBP, 0.1 M guanidinium thiocyanide and acetonitrile solvent. | ||||
| TBC | 4.15 | 0.730 | 0.718 | 2.17 |
| TMC | 2.06 | 0.634 | 0.750 | 0.98 |
| OMC | 4.96 | 0.730 | 0.743 | 2.69 |
| PC | 2.24 | 0.574 | 0.765 | 0.98 |
| CC | 2.63 | 0.577 | 0.731 | 1.11 |
| TBR | 1.40 | 0.537 | 0.775 | 0.58 |
| TMR | 0.813 | 0.510 | 0.779 | 0.32 |
| OMR | 2.50 | 0.525 | 0.775 | 1.02 |
| J SC (mA cm−2) | V OC (V) | ff | η (%) | |
|---|---|---|---|---|
| OMNC | 6.84 | 0.690 | 0.689 | 3.25 |
| CNC | 6.84 | 0.735 | 0.663 | 3.33 |
| HNC | 5.43 | 0.752 | 0.737 | 3.01 |
In Fig. 9b, the dark current curves of OMNC, CNC and HNC are displayed. The dark current values of dyes are in the order of HNC < OMNC ∼ CNC indicating that, on increase the VOC are beneficial to suppress the recombination of injected electrons at the interface or in the electrolyte.
6. Conclusions
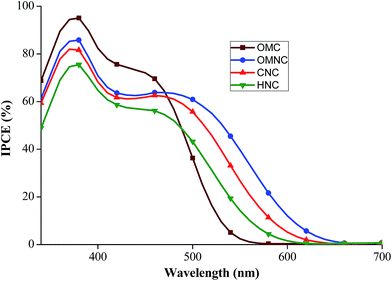
In conclusion, to understand the influence of donor groups on the optoelectronic properties, a series of carbazole based sensitizers with phenyl and amine based donor groups were successfully synthesized and characterized by 1H and 13C NMR, and mass spectrometric measurements. For phenyl based carbazole sensitizers, the maximum absorption appeared around 418 nm for methoxy substituted phenyl based donor (OMC) with CAA acceptor and 457 nm for RA as acceptor (OMR). In the presence of amine donor, CNC shows maximum absorption at 459 nm which reveals the more donating strength of amine donor substituted carbazole compounds than the phenyl donor based sensitizers. Due to H type aggregation of phenyl based carbazole dyes on the surface of TiO2, a hypsochromic shift is observed in the absorption spectrum of solid state films. From electrochemical data, the observed HOMO and LUMO values supports the feasible thermodynamic driving force for efficient dye regeneration and electron injection in DSSC. The DSSC made with these carbazole sensitizers displayed overall conversion efficiency in the range of 0.32–3.33%. OMC shows overall conversion efficiency of 2.69% (JSC = 4.96 mA cm−2, VOC = 0.730 mV, ff = 0.743) for phenyl based donor groups and CNC shows 3.33% (JSC = 6.84 mA cm−2, VOC = 735 mV, ff = 0.663) for amine based donor groups. RA acceptor based sensitizers shows overall poor performance due to inefficient electron injection of RA acceptor groups to TiO2. The maximum IPCE of OMNC, CNC and HNC at 460 nm are 64%, 62% and 56%, respectively. Despite the high IPCE (72% at 450 nm) of OMC than amine donor compounds, its lower absorbance region than amine based donor compounds results in lower efficiency. The open circuit potential is found to be depending on the bulkiness in the amine based donors which was probed through transient photovoltage measurements. The observed results provides vital information for future construction of donor fragments with more π-conjugated molecules to achieve wide range of absorption in the visible region. Overall, the results emphasize the varying strength and size of the donor fragment in the D–π–A sensitizers have significant impact in determining the optoelectronic and photovoltaic properties.Acknowledgements
S. J. (Ref. No. 039680/E15/2011 Dt: 17.02.2011) thanks UGC-BSR (RFSMS) for the Fellowship. G. P. thanks the Swiss government scholarship (Ref No. 2012.0795/India/OP). R. R. thanks DST-NM (Ref. No. SR/NM/NS-26/2013(G), DT: 21.10.2014) and UGC (MRP-MAJOR-CHEM-2013-35169) for the Project and UGC-Emeritus fellowship (UGC-EF-7855, 2016-2017). P.G. thank acknowledge funding from the European Union Seventh Framework Programme [FP7/2007-2013] under grant agreement no. 604032 of the MESO project. The present study was performed within the Project “Establishment of the Laboratory Photoactive Nanocomposite Materials” No. 14.Z50.31.0016 supported by a Mega-grant of the Government of the Russian Federation. Authors also thank UGC/DST-FIST for NMR facilities in the School of Chemistry, Bharathidasan University.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, F. E. CurchodBasile, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- (a) Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138–1141 CrossRef CAS; (b) K. Hara, M. Kurashige, Y. Danoh, C. Kasada, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, New J. Chem., 2003, 27, 783–785 RSC.
- B. Liu, W. Zhu, Q. Zhang, W. Wu, M. Xu, Z. Ning, Y. Xie and H. Tian, Chem. Commun., 2009, 13, 1766–1768 RSC.
- C. Zafer, M. Kus, G. Turkmen, H. Dincalp, S. Demic, B. Kuban, Y. Teoman and S. Icli, Sol. Energy Mater. Sol. Cells, 2007, 91, 427–431 CrossRef CAS.
- X. Ma, J. Hua, W. Wu, Y. Jin, F. Meng, W. Zhan and H. Tian, A high-efficiency cyanine dye for dye-sensitized solar cells, Tetrahedron, 2008, 64, 345–350 CrossRef CAS.
- S. Hayashi, M. Tanaka, H. Hayashi, S. Eu, T. Umeyama, Y. Matano, Y. Araki and H. Imahori, J. Phys. Chem. C, 2008, 112, 15576–15585 CAS.
- P. Shen, Y. Liu, X. Huang, B. Zhao, N. Xiang, J. Fei, L. Liu, X. Wang, H. Huang and S. T. Tan, Dyes Pigm., 2009, 83, 187–197 CrossRef CAS.
- D. Liu, B. Zhao, P. Shen, H. Huang, L. Liu and S. T. Tan, Sci. China, Ser. B: Chem., 2009, 52, 1198–1209 CrossRef CAS.
- F. Sanda, T. Nakai, N. Kobayashi and T. Masuda, Macromolecules, 2004, 37, 2703–2708 CrossRef CAS.
- (a) V. Promarak, A. Punkvuang, T. Sudyoadsuk, S. Jungsuttiwong, S. Saengsuwan, T. Keawin and K. Sirithip, Tetrahedron, 2007, 63, 8881–8890 CrossRef CAS; (b) S. Jagadeeswari, G. Paramaguru, S. Thennarasu and R. Renganathan, J. Mol. Struct., 2014, 1060, 191–196 CrossRef CAS.
- Z. S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993–4003 CrossRef CAS.
- S. Cai, X. Hu, J. Han, Z. Zhang, X. Li, C. Wang and J. Su, Tetrahedron, 2013, 69, 1970–1977 CrossRef CAS.
- M. Liangwab and J. Chen, Chem. Soc. Rev., 2013, 42, 3453–3488 RSC.
- (a) M. D. Zhang, H. X. Xie, X. H. Ju, L. Qin, Q. X. Yang, H. G. Zheng and X. F. Zhou, Phys. Chem. Chem. Phys., 2013, 15, 634–641 RSC; (b) T. Sudyoadsuk, S. Pansay, S. Morada, R. Rattanawan, S. Namuangruk, T. Kaewin, S. Jungsuttiwong and V. Promarak, Eur. J. Org. Chem., 2013, 5051–5063 CrossRef CAS; (c) N. Koumura, Z. S. Wang, M. Miyashita, Y. Uemura, H. Sekiguchi, Y. Cui, A. Mori, S. Mori and K. Hara, J. Mater. Chem., 2009, 19, 4829–4836 RSC; (d) S. Cai, G. Tian, X. Li, J. Su and H. Tian, J. Mater. Chem. A, 2013, 1, 11295–11305 RSC; (e) A. Venkateswararao, K. R. Justin Thomas, C.-T. Li and K.-C. Ho, RSC Adv., 2015, 5, 17953–17966 RSC.
- H. Tian, X. C. Yang, R. K. Chen, R. Zhang, A. Hagfeldt and L. Sun, J. Phys. Chem. C, 2008, 112, 11023–11033 CAS.
- B. C. O'Regan and F. J. Lenzmann, J. Phys. Chem. B, 2004, 108, 4342 CrossRef.
- P. Gao, Y. J. Kim, J. H. Yum, T. W. Holcombe, M. K. Nazeeruddin and M. Grätzel, J. Mater. Chem. A, 2013, 1, 5535–5544 CAS.
- W. H. Nguyen, C. D. Bailie, J. Burschka, T. Moehl, M. Grätzel, M. D. McGehee and A. Sellinger, Chem. Mater., 2013, 25, 1519–1525 CrossRef CAS.
- (a) K. R. J. Thomas, Y. C. Hsu, J. T. Lin, K. M. Lee, K. C. Ho, C. H. Lai, Y. M. Cheng and P. T. Chou, Chem. Mater., 2008, 20, 1830–1840 CrossRef CAS; (b) J. Song, F. Zhang, C. Li, W. Liu, B. Li, Y. Huang and Z. Bo, J. Phys. Chem. C, 2009, 113, 13391–13397 CrossRef CAS; (c) P. G. Hoertz, R. A. Carlisle and G. J. Meyer, Nano Lett., 2003, 3, 325–330 CrossRef CAS; (d) G. Paramaguru, R. Vijay Solomon, S. Jagadeeswari, P. Venuvanalingam and R. Renganathan, J. Photochem. Photobiol., A, 2013, 271, 31–44 CrossRef; (e) G. Paramaguru, R. Vijay Solomon, S. Jagadeeswari, P. Venuvanalinga and R. Renganathan, Eur. J. Org. Chem., 2014, 753–766 CrossRef CAS; (f) G. Paramaguru, A. Chandiran, P. Gao, R. Rajalingam, M. Grätzel and Md. K. Nazeeruddin, J. Phys. Chem. C, 2014, 118, 16896–16903 CrossRef.
- M. K. Nazeeruddin, A. K. Rodicio, R. Humpbry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- (a) A. Baheti, P. Singh, C. P. Lee, K. R. Justin Thomas and K. C. Ho, J. Org. Chem., 2011, 76, 4910–4920 CrossRef CAS PubMed; (b) G. Paramaguru, A. Chandiran, P. Gao, R. Rajalingam, M. Grätzel and Md. K. Nazeeruddin, ChemPhysChem, 2015, 16, 1035–1041 CrossRef PubMed.
- Y. Ooyama, Y. Hagiwara, Y. Oda, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2336–2340 RSC.
- (a) P. B. Pati and S. S. Zade, Tetrahedron, 2013, 69, 2167–2174 CrossRef CAS; (b) S. Jagadeeswari, G. Paramaguru, R. Madhumitha, Md. K. Nazeeruddin and R. Renganathan, J. Photochem. Photobiol., A, 2015, 299, 194–202 CrossRef.
- L. M. Peter, Phys. Chem. Chem. Phys., 2007, 9, 2630–2642 RSC.
- B. C. O'Regan and J. R. Durrant, Acc. Chem. Res., 2009, 42, 1799–1808 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure of carbazole intermediates and the characterization. See DOI: 10.1039/c6ra01185c |
| This journal is © The Royal Society of Chemistry 2016 |