Preparation and characterization of room temperature vulcanized silicone rubber using α-amine ketoximesilanes as auto-catalyzed cross-linkers†
Abstract
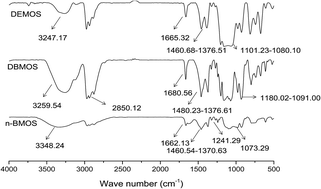
A series of α-amine ketoximesilanes were prepared and used as auto-catalyzed cross-linkers in one component room temperature vulcanized (RTV) silicone rubber. The synthesized α-amine ketoximesilanes were characterized by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR). Better performances of RTV silicone rubber including tack free time, viscosity, mechanical performances and thermal stability using α-amine ketoximesilanes as cross-linkers were successfully obtained, in comparison to those performances of RTV silicone rubber using commercial methyltris(methylethylketoxime)silane (MOS) as a cross-linker and dibutyltin dilaurate as a catalyst. Further, we applied density function theory (DFT) method to confirm α-amine ketoximesilanes plays a autocatalytic effect in the hydrolytic reaction of α-amine ketoximesilanes with H2O. The experimental results showed that using α-amine ketoximesilanes as cross-linkers is a practical way to obtain RTV silicone rubber with excellent properties, as well as avoiding using high chemical toxicology of organic metal catalyst in curing system.

 Please wait while we load your content...
Please wait while we load your content...