Growth kinetics of colloidal Ge nanocrystals for light harvesters†
Abstract
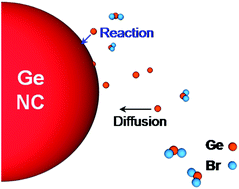
Colloidal Ge nanocrystals (NCs) are gaining increased interest because of their potential application in low-cost optoelectronic and light harvesting devices. However, reliable control of colloidal NC synthesis is often an issue and a deeper understanding of the key-role parameters governing NC growth is highly required. Here we report an extended investigation on the growth of colloidal Ge NCs synthesized from a one-pot solution based approach. A systematic study of the effects of synthesis time, temperature and precursor concentration is elucidated in detail. X-ray diffraction (XRD) analysis reveals the presence of crystalline Ge NCs with a mean size (from 5 to 35 nm) decreasing with the increase of precursor concentration. Such a trend was further confirmed by scanning electron microscopy (SEM) and dynamic light scattering (DLS) analysis. Moreover, the temporal NC size evolution shows a typical saturating behaviour, where characteristic time shortens at higher precursor concentration. All these growth features were satisfactorily simulated by a numerical NC growth model, evidencing that the kinetics of NC growth is controlled by a reaction-limited regime with typical activation energy of 0.7 eV. Finally, light absorption in the visible region and the successful realization of a hybrid photodetector, employing colloidal Ge NCs embedded in PEDOT:PSS polymer, showed the capability of low-cost colloidal Ge to act as light harvester. These results put new understanding for a reliable control of colloidal NC growth and the development of low-cost devices.

 Please wait while we load your content...
Please wait while we load your content...