Investigation on the interfacial properties of a viscoelastic-based surfactant as an oil displacement agent recovered from fracturing flowback fluid
Abstract
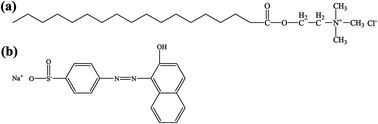
The utilization of a viscoelastic-based surfactant recovered from fracturing flowback fluid in chemical flooding was investigated in this paper. The interfacial tension behavior, wettability alteration, dynamic adsorption capacity and incremental oil recovery ability were studied. The oil/water interfacial tension was lowered to 10−4 to 10−3 mN m−1 due to 0.06 to 0.15 wt% of VES in the fracturing flowback fluid. The oil-wet surface can be easily converted to weak water-wet through adsorption of VES. A series of sandpack core flooding tests were conducted under the Dingbianluo reservoir condition to investigate the effects of interfacial tension and slug sizes on enhanced oil recovery. Finally, an SEM scanning test and microscopic displacement test were used to investigate the mechanism of surfactant flooding. This investigation confirmed that the fracturing flowback fluid recovered from fracturing treatment can be reused for surfactant flooding with probable environmental and economic benefits.

 Please wait while we load your content...
Please wait while we load your content...