Solid lipid nanoparticle enhances bioavailability of hydroxycitric acid compared to a microparticle delivery system
P. N. Ezhilarasiac,
S. P. Muthukumarb and
C. Anandharamakrishnan*ac
aCentre for Food Nanotechnology, CSIR-Central Food Technological Research Institute, Mysore-570 020, India. E-mail: c.anandharamakrishnan@gmail.com; Fax: +91 821 2517233; Tel: +91 821 2514310
bAnimal House Facility, CSIR-Central Food Technological Research Institute, Mysore-570 020, India
cAcSIR-Academy of Scientific and Innovative Research, CSIR-CFTRI Campus, Mysore-570 020, India
First published on 10th May 2016
Abstract
Solid lipid nanoparticles (SLN) are the most promising delivery system that improves the stability, bioavailability and controlled release of food bioactive compounds. The delivery of any bioactive compound to targeted sites is directly affected by particle size and thus a nano delivery system has the potential to enhance bioavailability, the controlled release of the bioactive compounds, to a greater extent than microencapsulation. However, the effect of different particle sizes on the bioavailability of the active compound has been less explored. Moreover, nanoencapsulation of hydrophilic compounds by SLN technique has not been much studied due to the complexity involved in non-compatibility between the hydrophilic core and lipophilic wall matrix. Hence, in order to improve the efficiency of SLN to load the hydrophilic molecules and to evaluate the effect of micro and nanoparticles on the bioavailability, HCA, a model hydrophilic compound was nanoencapsulated using solid lipid nanocarriers. The HCA loaded SLN (327 nm) exhibited an encapsulation efficiency of about 57% and excellent storage stability with retention of 82% HCA and 558 nm particle size during 50 days of storage. Moreover, in simulated gastrointestinal conditions, SLN-HCA exhibited higher gastrointestinal stability (about 88%) for HCA with better retention of particle size and it also showed excellent controlled release of HCA. On comparison of bioavailability, HCA loaded nanoparticles (SLN-HCA) had 2 fold higher bioavailability than unencapsulated HCA and 1.3 fold higher bioavailability than the microparticles (SLM-HCA) due to smaller particle size, longer residence time and controlled release of HCA.
Introduction
Nanotechnology has great potential for improving the delivery of bioactive compounds to improve human health. Nanoencapsulation is a technology to encapsulate substances in miniature and refers to bioactive packing at the nanoscale.1 Generally, nanocapsules are defined as particles of 1 to 1000 nm and have the potential to enhance bioavailability, controlled release and precision targeting of the bioactive compounds due to smaller particle size and larger surface area.2,3 In general, the physicochemical properties such as particle size, size distribution, surface area, shape, solubility, encapsulation efficiency, and releasing mechanisms can be altered by the encapsulation technique and delivery systems.Nano delivery systems such as lipid or natural polymers based capsules are most often utilized for nanoencapsulation of food bioactives.4,5 Lipid based delivery system (emulsions, liposomes and solid lipid nanoparticles) are composed of biocompatible and biodegradable material, had a prioritized attention in the food industry due to their non-toxicity, stability, easy adoptability for large scale production and cost effectiveness. Solid Lipid Nanoparticles (SLN) are the recent generation sub-micron colloidal carriers (ranging from 50 to 1000 nm), composed of solid lipid with the bioactives being a part of the lipid matrix and the particle is dispersed in water or in aqueous surfactant solution. SLN offers unique properties such as small size, large surface area, high drug loading, interaction of phases at the interface and potential to improve the performance of biomolecule.6 Moreover, it has flexibility in controlled release, slower degradation rate, sustained release and effective protection of biomolecule from chemical degradation.7 SLN have potential to encapsulate both lipophilic and hydrophilic compounds to enhance its applications in pharmaceutical, cosmetic and food industries as well.
The major problem of SLN is the capacity to load hydrophilic molecule is low due to its high tendency to migrate into the external aqueous phase and low affinity between hydrophilic core and lipid. Since the active compound has to be soluble and miscible with the lipid matrix to have higher encapsulation efficiency.6 In order to improve the loading efficiency of hydrophilic compound into solid lipid carriers, hydroxycitric acid (HCA) was chosen as a model compound. HCA is one of the major organic acid present in Garcinia cowa and attributed to cardio-protection, anti-diabetic effect, weight loss and reduces lipid abnormalities.8 However, the native form of HCA (free HCA available in fruit) is unstable, undergoes rapid lactonization during evaporation, drying and degrades in undesirable gastrointestinal condition. Thus, encapsulating the HCA can improve its stability9–11 and bioavailability to a large extent.
The nanoencapsulation has the potential to enhance bioavailability, controlled release and enable precision targeting of the bioactive compounds in a greater extent than microencapsulation.12 This is due to the difference in particle size that can have different surface area which in turn may influence the stability, bioaccessibility and bioavailability of the encapsulated compound.13 Hence there is a need to evaluate the effect of micro and nanoencapsulation of biomolecule on the stability and bioavailability of encapsulated molecule.
Hence, the objective of this study is to nanoencapsulate the HCA by solid lipid nanoparticles and compare its characteristics, storage stability, gastrointestinal stability, in vitro release and in vivo bioavailability with HCA loaded microparticles and unencapsulated HCA.
Materials and methods
Dried Garcinia cowa fruit rinds were obtained from Assam, India and HCA was isolated with 78% purity from Garcinia water extract by ion exchange chromatography. Glycerol monostearate (GMS), stearic acid (SA), soy lecithin and Tween 80 was acquired from Himedia (Mumbai, India). All other chemicals and reagents were of analytical grade.Preparation HCA loaded solid lipid nanoparticles, microparticles and unloaded nanoparticles
SLN-HCA were prepared by hot homogenization and ultrasonication method as described by Huang et al.14 with some modifications, using GMS and SA as lipid phase and Tween 80 as surfactant and soy lecithin as co-surfactant. Emulsifiers and a known quantity of HCA were added to the water phase. Both lipid and water phase were heated separately to 80 °C and homogenized together at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm with an Ultra Turrax T-25 homogenizer (IKA Labortechnik, Germany) for 5 min. The resultant emulsion was immediately subjected to high intensity probe sonication (60% amplitude) for 10 min at the same temperature (75 °C) and cooled immediately to 10 °C using an ice bath.
000 rpm with an Ultra Turrax T-25 homogenizer (IKA Labortechnik, Germany) for 5 min. The resultant emulsion was immediately subjected to high intensity probe sonication (60% amplitude) for 10 min at the same temperature (75 °C) and cooled immediately to 10 °C using an ice bath.
The various HCA loaded SLN-HCA formulations are prepared by above described method with various compositions as given in Table 1. Then the composition was optimised based on the particle size and encapsulation efficiency which were carried out as described below. Using the optimised composition, HCA loaded micro particles (SLM-HCA) and unloaded nanoparticles (SLN-unloaded) were prepared by the method as described above. The HCA loaded micro particles were prepared with slight modification in the procedure. Both water phase and lipid phase are heated to a temperature of 80 °C and continuously stirred using magnetic stirrer at 200 rpm for 5 min and immediately cooled using ice bath.
| Formulations | Lipid (mg) | Drug-HCA (mg) | Lipid to drug ratio | Surfactant (mg) | Co-surfactant (mg) | Surfactant to co-surfactant ration |
|---|---|---|---|---|---|---|
| F1 | 500 | 250 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
500 | 500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F2 | 500 | 250 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
750 | 500 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F3 | 500 | 250 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1000 | 500 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F4 | 1000 | 250 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
500 | 500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F5 | 1000 | 250 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
750 | 500 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F6 | 1000 | 250 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1000 | 500 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F7 | 1500 | 250 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
500 | 500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F8 | 1500 | 250 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
750 | 500 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F9 | 1500 | 250 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1000 | 500 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F10 | 2000 | 250 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
500 | 500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F11 | 2000 | 250 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
750 | 500 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| F12 | 2000 | 250 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1000 | 500 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Characterisation of solid lipid particles
Storage stability of solid lipid particles
The colloidal dispersions of SLN and SLM particles were separately stored at room temperature (27 °C) and refrigerated temperature 4 °C for period of 50 days. The physiochemical stability of the SLN, SLM formulations was evaluated through particle size analysis, and HCA content by RP-HPLC method at time interval of 10 days.Gastrointestinal stability of solid lipid particles
The gastrointestinal stability of SLN, SLM and unencapsulated HCA were analysed in simulated gastric and intestinal condition using the method described by Bermudez-Soto et al.19 with slight modifications. A known quantity of solid lipid nanoparticles, solid lipid microparticles and unencapsulated HCA were suspended separately in 20 ml of simulated gastric fluid (2 g l−1 of NaCl with their pH adjusted to 2 using 5 M HCL solution and 0.3% pepsin). The samples were incubated at 37 °C in a shaking water bath for 2 h. After the gastric digestion, the pH of the digesta was brought to 7.5 with 1 N NaHCO3, intestinal enzyme complex (pancreatin 4.8 mg ml−1, bile salt 5 mg ml−1, lipase 0.4 mg ml−1) and calcium chloride solution 750 mM. Then samples were incubated in a shaking water bath at 37 °C for 2 h for intestinal digestion. An aliquots of samples (1 ml) were taken at 0, 1 h, 2 h, 3 h and 4 h then reaction was stopped by cooling them in ice and analysed for HCA content by RP-HPLC method. The digesta sample collected at the end of gastric digestion (2 h) and intestinal digestion (4 h) was subjected to particle size analysis to evaluate the physical stability of these formulations in simulated gastrointestinal conditions.In vitro release of HCA in solid lipid particles
In vitro release of colloidal dispersions of SLN, SLM and unencapsulated HCA were performed separately using the dialysis bag technique to study their release kinetics as described by Fathi et al.20 with some modification. For in vitro release study, the free HCA molecules has be separated from the SLN particles for quantification and many studies had reported that dialysis bag of 12–14 kDa MWCO was suitable for in vitro release study.20–22 The phosphate buffered saline (PBS; 100 mM, pH 7.4) was used as the release medium. The dialysis bag (molecular weight cut off: 12–14 kDa, Himedia, Mumbai, India) was soaked in distilled water for 12 hours prior to use. 1 ml aliquot of prepared HCA loaded lipid formulation and unencapsulated HCA was sealed in the dialysis bag and immersed in 50 ml of PBS release medium. The samples were placed in a thermostatic shaker at 37 °C and 100 rpm. An aliquot of 5 ml of release medium was withdrawn at pre-determined time points such as 0, 0.5, 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 24 h and replaced immediately with the same volume of fresh PBS to maintain the sink conditions. The HCA release at various time intervals was quantified using RP-HPLC method. The cumulative percentage of released compound was determined.In vivo bioavailability of solid lipid particles
In vivo bioavailability study was carried out by animal experiments according to the CPCSEA guidelines and also in compliance with the relevant laws and institutional guidelines. Moreover, the experimental method was approved by institutional (CFTRI) animal ethical committee. Male Wistar rats were obtained and quarantined in the animal house, maintained at 25 ± 2 °C and 50–60% RH. A 12 h dark/light cycle was maintained throughout the study. Rats had free access to food (pellet diet supplied from M/s Petcare India Ltd., Bangalore) and water ad libitum. Rats (100 ± 10 g) were divided into three groups of 30 each. Each group received either unencapsulated HCA or SLN-HCA or SLM-HCA at dose equivalent to 350 mg kg−1 of HCA by oral gavage feeding. From the time, the rats received dosage, blood samples (0.5 ml) were collected from their retro-orbital plexus at predetermined intervals of 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 10 h. From the blood samples, plasma was immediately separated by centrifugation (3000 rpm for 15 min) and stored at −80 °C until further analysis and the HCA content was quantified by RP-HPLC method.Statistical analysis
The statistical analysis were carried out by one way ANOVA using the Minitab 17 software (Minitab Ltd, USA). The differences among the means at p < 0.05 were considered as significant.Results and discussion
Optimisation of HCA loaded SLN formulation
The HCA loaded solid lipid nanoparticle (SLN-HCA) formulation was optimised by varying the composition based on the lipid to drug ratio and surfactant to co-surfactant ratio. The average particle size and encapsulation efficiency of the formulations are given in Table 2.| Formulations | Lipid to drug ratio | Surfactant to co-surfactant ratio | Particle size (nm) | Encapsulation efficiency (%) |
|---|---|---|---|---|
| a a–k The values are expressed as mean ± standard deviation (no. of replications = 3). Mean with superscripts of different lowercase letters along the column are significantly different (p < 0.05). | ||||
| F1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 ± 7a | 10.7 ± 0.52a |
| F2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
56 ± 3b | 12.5 ± 0.86a |
| F3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 ± 12c | 14.4 ± 0.78a |
| F4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
256 ± 8d | 28.5 ± 1.23b |
| F5 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
205 ± 5e | 35.6 ± 0.89b |
| F6 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
178 ± 7f | 40.6 ± 0.56c |
| F7 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
423 ± 10g | 45.8 ± 0.54d |
| F8 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
386 ± 7h | 50.6 ± 0.79e |
| F9 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
327 ± 5i | 57.3 ± 1.28f |
| F10 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
633 ± 2j | 52.5 ± 1.42g |
| F11 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
587 ± 5k | 55.4 ± 0.89g,h |
| F12 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
510 ± 10k | 57.2 ± 0.78g,h |
The values are expressed as mean ± standard deviation (no. of replications = 3). Mean with superscripts of different small letters in the same columns are significantly different (p < 0.05).
Increase in lipid to drug ratio from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shown significant (p < 0.05) increase the particle size from 42 to 633 nm. This is because, higher lipid content in the formulation requires high stabilizer concentrations to reduce the surface tension and facilitate the particle size reduction. Among the formulations with same lipid to drug ratios, the increase in surfactant content (Tween 80) reduced the particle size. Since, higher surfactant reduce interfacial tension more effectively, facilitates the homogenisation of lipids in the aqueous phase and leads to smaller particles.23,24 Besides the Tween 80, its combination with the co surfactant, soy lecithin could have also helped to reduce the aggregation of the SLN and resulted in smaller particle size.25
1 shown significant (p < 0.05) increase the particle size from 42 to 633 nm. This is because, higher lipid content in the formulation requires high stabilizer concentrations to reduce the surface tension and facilitate the particle size reduction. Among the formulations with same lipid to drug ratios, the increase in surfactant content (Tween 80) reduced the particle size. Since, higher surfactant reduce interfacial tension more effectively, facilitates the homogenisation of lipids in the aqueous phase and leads to smaller particles.23,24 Besides the Tween 80, its combination with the co surfactant, soy lecithin could have also helped to reduce the aggregation of the SLN and resulted in smaller particle size.25
Besides the particle size, encapsulation efficiency is one of the most important characteristics to have bioactive enriched SLN. An increase in the lipid to core ratio from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited significant (p < 0.05) increase in encapsulation efficiency from 10 to 57%. Moreover, ratios at 6
1 exhibited significant (p < 0.05) increase in encapsulation efficiency from 10 to 57%. Moreover, ratios at 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had higher encapsulation efficiency due to higher lipid content that provides more space to incorporate the active compound and also reduces the escape of core compound to the external phase. However, beyond certain lipid to drug ratio (6
1 had higher encapsulation efficiency due to higher lipid content that provides more space to incorporate the active compound and also reduces the escape of core compound to the external phase. However, beyond certain lipid to drug ratio (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the encapsulation efficiency was not improved (in 8
1), the encapsulation efficiency was not improved (in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) much due to its limitations in the core accommodation (statistically insignificant). Moreover, increase in the surfactant ratio also increased the encapsulation efficiency and significantly (p < 0.05) in 6
1 ratio) much due to its limitations in the core accommodation (statistically insignificant). Moreover, increase in the surfactant ratio also increased the encapsulation efficiency and significantly (p < 0.05) in 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 lipid to drug ratio. Since, high concentration of Tween 80 increase the thickness of hydrophilic coating that helps to disperse the hydrophilic compound.26 The lipid to drug ratio of 6
1 lipid to drug ratio. Since, high concentration of Tween 80 increase the thickness of hydrophilic coating that helps to disperse the hydrophilic compound.26 The lipid to drug ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with surfactant to co-surfactant ratio of 2
1 with surfactant to co-surfactant ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave the highest encapsulation efficiency of around 57%.
1 gave the highest encapsulation efficiency of around 57%.
Based on the higher encapsulation efficiency and lower particle size, formulation F9 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, lipid: drug ratio with 2
1, lipid: drug ratio with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 surfactant: co-surfactant ratio) which had particle size around 327 nm and encapsulation efficiency of 57.3% was considered as the best formulation. Using the composition of optimised formulation F9, HCA loaded solid nanoparticles (SLN-HCA) HCA loaded solid microparticles (SLM-HCA) and unloaded nanoparticles (SLN-unloaded) were prepared and compared by characterization, stability and bioavailability studies.
1 surfactant: co-surfactant ratio) which had particle size around 327 nm and encapsulation efficiency of 57.3% was considered as the best formulation. Using the composition of optimised formulation F9, HCA loaded solid nanoparticles (SLN-HCA) HCA loaded solid microparticles (SLM-HCA) and unloaded nanoparticles (SLN-unloaded) were prepared and compared by characterization, stability and bioavailability studies.
Particle size analysis
The average particle size of SLN-HCA is 327 nm, SLM-HCA had 3.74 μm. Both SLN and SLM had narrow, monomodal and uniform size distribution. However, SLN (unloaded) had bimodal size distribution and larger particle size (380 nm). Even though there was no core compound inside the particles, SLN-unloaded are significantly (p < 0.05) larger in particle size than the SLN-HCA, which was contradictory to few reports27,28 and this could be due to the aggregation of the SLN (unloaded). This difference in particle size due to loading of HCA could be caused by the difference in pH of the external medium.The SLN-HCA and SLM-HCA had slightly acid pH around 3.7, whereas SLN (unloaded) had pH around 5.7. This slight acidic pH is due to the acid nature of unencapsulated HCA (pH-1.7) surrounding in the external medium. This difference in pH could have led to the slight aggregation of SLN-unloaded.
Morphology of solid lipid particles
The morphology of SLN-HCA, SLM-HCA and SLN (unloaded) are shown in Fig. 1a–c. Morphology shows that particles are spherical in shape with smooth surface, and not much aggregation. Moreover there was not much difference in the morphology of the particles due to the loading of HCA. All the three formulations were freeze dried using trehalose and their morphology is shown in Fig. 1d–f. It shows that particles have some irregularities at the periphery, with more aggregation. | ||
| Fig. 1 Morphology of (a)–(c) solid lipid particles and (d)–(f) freeze dried solid lipid particles by scanning electron microscopy. | ||
Since, lyophilization changes the properties of the surfactant layer due to water removal and increases the particle concentration that leads to aggregation. In this study, trehalose was used as a lyoprotectant to decrease nanoparticle aggregation. These freeze dried particles were re-dispersed in water and evaluated for particle size distribution. The average particle size of SLN-HCA and SLM-HCA are 592 nm and 4.2 μm. The SLN (unloaded) had particle size of about 726 nm. Even though the morphology of freeze dried particles showed that they are aggregated, but still they could retain the nano size on redispersion in water.
Thus the trehalose as a cryoprotectant had effectively reduce the aggregation of the particles. Lyophilization improves the physicochemical stability of lipid nanoparticles over the extended period of time and offers possibilities for SLN to be incorporated into pellets, tablets or capsules.
Encapsulation efficiency of HCA loaded lipid particles
SLN-HCA had encapsulation efficiency of about 57.3% and slightly higher than the reported studies on hydrophilic compounds loaded into SLN, which was around 41% and less than that.27–29 This high encapsulation efficiency may be due to several factors including nature of the lipid matrix as well as the surfactant and their interaction during preparation. Stearic acid is a self-emulsifying lipid and can entrap more hydrophilic compound in the particles. Moreover, the higher lipid content provides the additional space to accommodate active molecules to entrap in their matrix.26 Moreover, soy lecithin and Tween 80 helps to disperse the molecules of low lipophilicity in the lipid matrix due to a strong binding of phospholipids to the lipid that immobilize the interfacial film and diminish the repulsion between the hydrophilic core and wall.26,30 However, on comparison HCA loaded micro and nano particles, micro particles (52.8%) had significantly (p < 0.05) lower encapsulation efficiency than the nano particles. Moreover, the HCA could be loaded into SLN as a matrix type, as the hydrophilic bioactives can be dispersed throughout the fat phase and forms fine solid particles.31DSC study
The DSC provides information about the physical state and the degree of crystallinity of SLN by thermal behaviour which is important for its performance in both in vitro and in vivo.32The DSC thermograms of bulk lipid, mixture of lipids and lipid particles are given in Fig. 2. The thermal behaviour of the untreated bulk lipid was chosen as reference. The pure GMS exhibited maximum endothermic peak at 60.08 °C and stearic acid exhibited at 61.23 °C. The melting points of SLN-HCA and SLM-HCA are 56.78 °C and 56.66 °C, respectively. Moreover, SLN (unloaded) had slightly higher melting point around 57.32 °C. When compared to bulk lipid, the melting point of SLN/SLM is reduced to about 3–4 °C and peaks of the heating thermograms are broadened. This decrease in melting point of colloidal systems can be assigned to the colloidal dimensions of the particles with large surface to volume ratio and small size effect along with the interactions of lipids and emulsifier that generates the surface tension over the particle surface. The broadening of the heating peak and the reduction of the melting point indicate an increased number of lattice defects and favourable for encapsulating more active molecules.33 Moreover, there was additional peak in the thermogram of mixture, at 95.98 °C, which is due to the soy lecithin in the formulation.
Storage stability of solid lipid dispersions
The physiochemical stability of HCA loaded nanoparticles, microparticles and unloaded nanoparticles during the storage period of 50 days was evaluated at room and refrigerated temperature. The storage stability was evaluated through particle size analysis, and HCA content at interval of 10 days. | ||
| Fig. 3 Particle size of solid lipid particles during the storage period. Error bar represents the standard deviation of means (n = 3). | ||
However, the SLN-unloaded had poor aggregation stability (increased from 382 to 1783 μm) than the HCA loaded SLN and SLM. Within 10 days itself, the SLN-unloaded had shown maximum aggregation of particles which can be due to the pH difference of the external medium. Since SLN/SLM had pH of about 3.7 and SLN (unloaded) had pH of 5.7 initially. This difference in pH could have altered the repulsive force of the system and resulted in aggregation in SLN (unloaded). Similarly, SLM-HCA suspension had increase (statistically insignificant) in particle size of about 1.05 fold (from 3.74 to 3.95 μm) at refrigerated temperature and 1.11 fold (3.74 to 4.18 μm, statistically insignificant) at room temperature.
This increase in particle size may be due to a morphological changes (needle-like structures formation) caused by polymorphic transition of SLN from thermodynamically more unfavorable α-crystal modification into the β-form, during storage. However, SLN-HCA could still retain the particle size within the nano range at both room and refrigeration condition. The high stability of this system can be attributed to strong steric repulsion between the lipid nanoparticles generated by Tween 80 as well its combination with soy lecithin that efficiently prevented the particle agglomeration and resulted in better storage stability.34 Moreover the longer chain triglycerides based lipids (stearic acid and GMS) has less rate of polymorphic transitions than the shorter chain triglycerides35 and shows better stability. This indicates the excellent physical stability of HCA loaded nano and micro particles towards aggregation during storage.
 | ||
| Fig. 4 Chemical stability of HCA and HCA loaded solid lipid particles during the storage period. Error bar represents the standard deviation of means (n = 3). | ||
During the storage period, initially there was a slight expulsion of the compound from SLN/SLM-HCA due to the complex crystallization behaviour related to the polymorphic transitions during the storage period. During polymorphic transition, the lipid tends to crystallize to more perfect crystalline β-modifications that ultimately lead to expulsion of the bioactives from the crystal matrix.36 After 20 days, there was conversion of expelled HCA to HCA lactone. However, when compared to unencapsulated HCA, SLN/SLM had shown excellent chemical stability of HCA during the storage period.
Stability of solid lipid particles in simulated gastro intestinal conditions
 | ||
| Fig. 5 Stability of solid lipid particles in simulated gastrointestinal conditions. (a) Particle size; (b) gastrointestinal stability-HCA content. | ||
However, at the end of intestinal digestion, the particle size of SLN/SLM formulations had increased. The SLN-HCA had shown 1.67 fold (452 nm to 758 nm) increase in particle size. Similarly the SLM-HCA and SLN (unloaded) had shown 1.51 fold (4800 to 7260 nm) and 2.41 fold (435 to 1050 nm) increase in particle size respectively. These increase in particle was statistically significant.
This increase in size may be due to desorption of surfactant layer from particles by the action of bile salts and phospholipids leading to particle aggregation and exposing the nanoparticle surface for lipid hydrolysis by intestinal enzyme complex. Moreover, salts may reduce repulsive interactions between droplets by screening electrostatic forces, and promotes bridging flocculation between one particles to another. However, the strong steric repulsion due to interfacial layer by Tween 80 around the lipid particles was large enough to overcome the attractive interactions in gastric conditions, but it was reduced in the intestinal conditions for all the formulations.
Hence, during the gastric and intestinal digestions, HCA remained chemically stable, without much aggregation of the particles. Therefore HCA retains in active as well as nano form in the intestine during absorption. This indicates the solid lipid carrier as prominent delivery system suitable for oral administration.
In vitro release study
The in vitro release behavior of SLN-HCA, SLM-HCA and unencapsulated HCA in PBS (pH 7.4 at 37 °C) was studied using a hydrophilic dialysis membrane. The cumulative release profile of the formulations is shown in Fig. 6.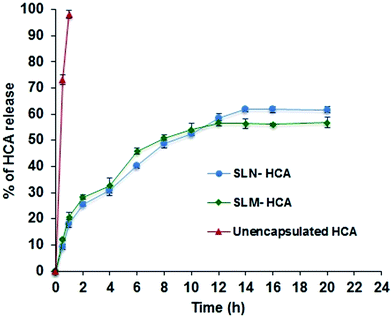 | ||
| Fig. 6 In vitro release profile of HCA from solid lipid particles. Error bar represents the standard deviation of means (n = 3). | ||
The unencapsulated HCA exhibited 100% release in first hour. SLN-HCA and SLM-HCA showed a biphasic pattern; a burst release of about 9–12% of the HCA in the first half an hour due to the rapid release of HCA molecules that are adsorbed to the particle surface, then a slow and sustained release of the loaded HCA. As the time progress, the water penetrates into system and causes swelling of matrix followed by the conversion of solid lipid into rubbery matrix, and then the diffusion of compound takes place.40
The SLN-HCA reached plateau in the release profile by 14th h and SLM-HCA exhibited by 12th h. In 14 h, SLN released 61% of HCA, SLM exhibited 56% of HCA release indicating the higher (statistically significant) release by SLN. The slow and sustained release by SLN and SLM for longer duration was probably due to the slow diffusion of HCA from the lipid matrix. The release of compounds depends upon the loading efficiency of compound and particle size, emulsifier coating over the particles. The release rate of both SLN and SLM was slightly less (not beyond 61%) which can be due to the less amount of HCA loaded into the particles and also due to the Tween 80 coating that could have increased the length of diffusion and decreased the release rate. Pandita et al.41 reported that increase in emulsifier concentration of SLN, reduced the core release due to the higher emulsifier thickness. Moreover, increase in lipid concentration could have also prolonged the release of HCA from the solid lipid matrix.21 This prolonged release behavior was desirable to protect the encapsulated compound from gastric and intestinal degradation.
However, few SLN studies showed sustained drug release upto several days. In this study, the drug release for about only 22 h was observed, which can be due to the hydrophilic core molecule HCA. Since the hydrophilic bioactive components can be held inside the particles by steric hindrance effects by molecular interactions (attractive forces between the bioactive and biopolymer molecules) and that controls the release of active compound.31 The HCA, being a hydrophilic molecule, could have been loosely bound to the lipid matrix due to less miscibility between them and resulted in rapid drug release in 22 h. Moreover, there are few studies that reported drug release for less than 25 h. 21,22,27,29,42
In vivo oral bioavailability
The three different formulations of HCA such as unencapsulated HCA, HCA loaded nanoparticles (SLN-HCA) and microparticles (SLM-HCA) were orally administered to rats in similar dosage of HCA i.e., 350 mg kg−1 body weight. Then the plasma concentration–time curves of HCA for all the three formulations were established and shown in Fig. 7 and the corresponding pharmacokinetic parameters are listed in Table 3. Peak plasma concentration (Cmax) of unencapsulated HCA was 11.77 μg ml−1 at 1.5 h. Likely, Cmax of SLN-HCA and SLM-HCA formulations are 15.54 and 13.66 μg ml−1, respectively. Comparitively, SLN-HCA and SLM-HCA displayed significantly (p < 0.05) higher values of Cmax than unencapsulated HCA.| Parameter | Unencapsulated HCA | SLN-HCA | SLM-HCA |
|---|---|---|---|
| a a–c The values are expressed as mean ± standard deviation (no. of replications = 3). Mean with superscripts of different lowercase letters along the column are significantly different (p < 0.05). | |||
| Dosage (mg kg−1) | 350 | 350 | 350 |
| Particle size | — | 327 nm | 3.7 μm |
| Cmax (μg ml−1) | 11.77 ± 0.99a | 15.54 ± 1.56b | 13.66 ± 1.23c |
| Tmax (h) | 1.5 | 2 | 2 |
| MRT (h) | 6 | 10 | 10 |
| AUC μg ml−1 | 27.02 | 55.61 | 42.32 |
| Relative bioavailability F1 | 1 | 2.058 | 1.566 |
| Relative bioavailability F2 | — | 1.314 | 1 |
Moreover, SLN-HCA had higher Cmax value than the SLM-HCA. Similarly, Tmax the time to achieve maximum plasma concentration of unencapsulated HCA was 1.5 h and for SLN-HCA and SLM-HCA, Tmax was increased from 1.5 h to 2 h. This delayed Tmax can be due to the encapsulation of HCA by the solid lipid matrix that had resulted in slow and sustained of HCA from the lipid matrix. The maximum residence times (MRT) for SLN-HCA and SLM-HCA for about 10 h, where unencapsulated HCA had MRT of about 6 h only. This shows the controlled release of HCA from solid lipid matrix.
Rats administered with SLN-HCA and SLM-HCA exhibited higher plasma HCA concentrations than the unencapsulated HCA at various time intervals. Even though SLN and SLM-HCA had almost same MRT, at all point SLN-HCA exhibited higher plasma HCA concentrations than the SLM-HCA. The area under the curve (AUC) which indicates the bioavailability of the compound for the given dose in the formulation is indicated in Table 2. The AUC 0 → t values of unencapsulated HCA was 27.02 μg ml−1. Similarly, the AUC values of SLN-HCA is 55.61 μg ml−1 and SLM-HCA is 42.32 μg ml−1. Peak plasma concentrations and AUC values were in the following order SLN-HCA > SLM-HCA > unencapsulated HCA. The significant increase (p < 0.05) in the AUC value of the SLN and SLM-HCA in comparison to unencapsulated HCA distinctly indicates the improved bioavailability of HCA in solid lipid carrier. Compared to unencapsulated HCA, SLN-HCA had 2 fold higher bioavailability and SLM-HCA had 1.5 fold higher bioavailability. This shows the higher absorption and bioavailability of SLN and SLM-HCA than the unencapsulated HCA. This enhancement in oral bioavailability of HCA by the lipid nanocarrier matrix can be explained by several mechanisms.
Relatively slow increase and higher plasma HCA concentration for a longer period of time were observed for SLN and SLM, with significantly delayed Cmax at 2 h, suggesting an obvious sustained release of the HCA from the lipid matrix. Moreover, SLN/SLM could protect the compound from chemical as well as enzymatic degradation. This can be due to the slower degradation rate of long chain fatty acids in the lipid matrix (GMS and stearic acid) that results in slow release of compound from the SLN matrix. Manjunath and Venkateswarlu43 also reported higher AUC and lower clearance from SLNs with longer chain length lipid. Moreover, the emulsifier Tween 80 could have provided coating over the core compound and protected the core from chemicals and enzymatic degradation with less particle aggregation until absorbed. Besides this, Tween 80 and lecithin, also enhance their permeation through the intestine, owing to the high affinity between lipid particles and the intestinal membrane.44 Meanwhile, unencapsulated HCA could have prone to degradation in GI conditions and then rapidly cleared from the systemic circulation.
Moreover, SLN-HCA had 1.3 fold higher bioavailability than the SLM-HCA. Due to their smaller particle size, the SLN may adhere to the gastrointestinal tract wall or enter the intervillar spaces thus increasing their residence time and in turn enhance absorption.45 This enables the more sustained release of the compound. Moreover, the HCA encapsulated in SLN form could be uptaken through the GI tract, where the particle size played a dominant role in absorption rate.46 The mechanisms of such uptake include diffusion of particles through mucus and accessibility to enterocyte surface, epithelial interaction and cellular trafficking, and exocytosis and systemic dissemination. The size of SLNs in the range of 20–500 nm allows the efficient uptake in intestine, particularly in the lymphoid sections of this tissue.47 Moreover, M cell uptake of particles was found to be size-dependent and higher uptake for the smaller particle size.48 Li et al.49 also reported 5.7 times higher bioavailability for the quercetin loaded nanoparticles than the quercetin suspension.
Hence, the HCA in nano form (SLN-HCA) could provide sustained release of HCA with a long circulation effect that prolonged the residence time in systematic circulation and resulted in higher bioavailability than the microparticles (SLM-HCA) and unencapsulated HCA. This increase in oral bioavailability of HCA can exhibit effective bioactivity of HCA at a lower amount.
Conclusion
The nanoencapsulation of HCA by SLN technique exhibited particle size of about 327 nm with 57% encapsulation efficiency. SLN-HCA showed excellent storage stability (82%) and gastrointestinal stability (88%) of HCA than unencapsulated HCA along with the good physical stability. On comparison of bioavailability HCA loaded nanoparticles (SLN-HCA) had 2 fold higher bioavailability than unencapsulated HCA and 1.3 fold higher bioavailability than the microparticles (SLN-HCA) due to smaller particle size, longer residence time and controlled release of HCA. Therefore, the nanoencapsulation by SLN technique is a smart delivery system suitable for any bioactive/drug compounds with multiple applications in food, pharmaceutical and nutraceutical industries.Acknowledgements
Authors wish to thank Prof. Ram Rajasekharan, Director, CSIR-CFTRI for the valuable support and help. P. N. Ezhilarasi thanks University Grant Commission for awarding Senior Research Fellowship.References
- A. Lopez, R. Gavara and J. Lagaron, Trends Food Sci. Technol., 2006, 17, 567–575 CrossRef.
- C. Anandharamakrishnan, Springer Briefs in Food, Health, and Nutrition, 2014 Search PubMed.
- P. N. Ezhilarasi, P. Karthik, N. Chhanwal and C. Anandharamakrishnan, Food Bioprocess Technol., 2013, 6, 628–647 CrossRef CAS.
- J. A. Bhushani and C. Anandharamakrishnan, Trends Food Sci. Technol., 2014, 38, 21–33 CrossRef.
- P. Karthik, P. N. Ezhilarasi and C. Anandharamakrishnan, Crit. Rev. Food Sci. Nutr., 2015 DOI:10.1080/10408398.2015.1006767.
- R. H. Muller and S. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161–177 CrossRef CAS PubMed.
- A. Saupe and T. Rades, Frontiers of nanotherapy, Springer, Dordrecht, 2006, pp. 41–50 Search PubMed.
- B. S. Jena, G. K. Jayaprakasha and K. K. Sakariah, J. Agric. Food Chem., 2002, 50, 3431–3434 CrossRef CAS PubMed.
- D. S. Pillai, P. Prabhasankar, B. S. Jena and C. Anandharamakrsishnan, Int. J. Food Prop., 2012, 15, 590–604 CrossRef CAS.
- S. Parthasarathi, P. N. Ezhilarasi, B. S. Jena and C. Anandharamakrishnan, Food Bioprod. Process., 2013, 91, 103–110 CrossRef CAS.
- P. N. Ezhilarasi, D. Indrani, B. S. Jena and C. Anandharamakrishnan, J. Sci. Food Agric., 2014, 94, 1116–1123 CrossRef CAS PubMed.
- J. Mozafari, L. Flanagan, A. Matia-Merino, A. Awati, Z. E. Omri, H. Suntres and H. Singh, J. Sci. Food Agric., 2006, 86, 2038–2045 CrossRef.
- E. C. Goethals, A. Elbaz, A. L. Lopata, S. K. Bhargava and V. Bansal, Langmuir, 2013, 29, 658–666 CrossRef CAS PubMed.
- X. Huang, Y.-J. Chen, D.-Y. Peng, Q.-L. Li, X.-S. Wang, D.-L. Wang and W.-D. Chen, Colloids Surf., B, 2013, 102, 391–397 CrossRef CAS PubMed.
- M. N. Patel, S. Lakkadwala, M. S. Majrad, E. R. Injeti, S. M. Gollmer, Z. A. Shah, S. H. S. Boddu and J. Nesamony, AAPS PharmSciTech, 2014, 15, 1498–1508 CrossRef CAS PubMed.
- K. Hippalgaonkar, G. R. Adelli, K. Hippalgaonkar, M. A. Repka and S. Majumdar, J. Ocul. Pharmacol. Ther., 2013, 29, 216–228 CrossRef CAS PubMed.
- A. Siddiqui, V. Gupta, Y.-Y. Liu and S. Nazzal, Int. J. Pharm., 2012, 431, 222–229 CrossRef CAS PubMed.
- P. N. Ezhilarasi, D. Indrani, B. S. Jena and C. Anandharamakrishnan, J. Food Eng., 2013, 117, 513–520 CrossRef CAS.
- M. J. Bermúdez-Soto, F. A. Tomás-Barberán and M. T. García-Conesa, Food Chem., 2007, 102, 865–874 CrossRef.
- M. Fathi, J. Varshosaz, M. Mohebbi and F. Shahidi, Food Bioprocess Technol., 2013, 6, 1464–1475 CrossRef CAS.
- S. Jose, S. S. Anju, T. A. Cinu, N. A. Aleykutty, S. Thomas and E. B. Souto, Int. J. Pharm., 2014, 474, 6–13 CrossRef CAS PubMed.
- N. P. Aditya, A. S. Macedo, S. Doktorovova, E. B. Souto, S. Kim, P.-S. Chang and S. Ko, LWT--Food Sci. Technol., 2014, 59, 115–121 CrossRef CAS.
- C. Zhang, C. Gu, F. Peng, W. Liu, J. Wan, H. Xu, C. W. Lam and X. Yang, Molecules, 2013, 18, 13340–13356 CrossRef CAS PubMed.
- F. Liu, J. Yang, L. Huang and D. Liu, Pharm. Res., 1996, 13, 1642–1646 CrossRef CAS.
- A. Fundaro, R. Cavalli, A. Bargoni, D. Vighetto, G. P. Zara, G. Paolo and M. R. Gasco, Pharmacol. Res., 2000, 42, 337–343 CrossRef CAS PubMed.
- Q. Lv, A. Yu, Y. Xi, H. Li, Z. Song, J. Cui, F. Cao and G. Zhai, Int. J. Pharm., 2009, 372, 191–198 CrossRef CAS PubMed.
- S. Singh, A. K. Dobhal, A. Jain, J. K. Pandit and S. Chakraborty, Chem. Pharm. Bull., 2010, 58, 650–655 CrossRef CAS PubMed.
- M. Shah, Y. K. Agrawal, K. Garala and A. Ramkishan, Indian J. Pharm. Sci., 2012, 74, 434–442 CrossRef CAS PubMed.
- M. Ghadiri, S. Fatemi, A. Vatanara, D. Doroud, A. R. Najafabadi, M. Darabi and R. A. Abbas, Int. J. Pharm., 2012, 424, 128–130 CrossRef CAS PubMed.
- M. A. Schubert and C. C. Muller-Goymann, Eur. J. Pharm. Biopharm., 2005, 61, 77–86 CrossRef CAS PubMed.
- D. J. McClements, Adv. Colloid Interface Sci., 2015, 219, 27–53 CrossRef CAS PubMed.
- W. Mehnert and K. Mader, Adv. Drug Delivery Rev., 2001, 47, 165–196 CrossRef CAS PubMed.
- B. Siekmann and K. Westesen, Colloids Surf., B, 1994, 3, 159–175 CrossRef CAS.
- S. Cai, Q. Yang, T. R. Bagby and M. L. Forrest, Adv. Drug Delivery Rev., 2011, 63, 901–908 CrossRef CAS PubMed.
- H. Bunjes, K. Westesen and M. H. J. Koch, Int. J. Pharm., 1996, 129, 159–173 CrossRef CAS.
- R. H. Muller, M. Radtke and S. A. Wissing, Int. J. Pharm., 2002, 242, 121–128 CrossRef CAS PubMed.
- E. Zimmermann and R. H. Muller, Eur. J. Pharm. Biopharm., 2001, 52, 203–210 CrossRef CAS PubMed.
- G. R. Jose, D. Josephine and S. A. Rios, US Pat., 6,596,308, 2003.
- C. Olbrich and R. H. Müller, Int. J. Pharm., 1999, 180, 31–39 CrossRef CAS PubMed.
- A. R. Gardouh, S. Gad, H. M. Ghonaim and M. M. Ghorab, Br. J. Pharm. Res., 2013, 3, 326–346 CrossRef.
- D. Pandita, A. Ahuja, T. Velpandian, V. Lather, T. Dutta and R. K. Khar, Pharmazie, 2009, 64, 301–310 CAS.
- M. Yasira and U. V. S. Sara, Acta Pharm. Sin. B, 2014, 4, 454–463 CrossRef PubMed.
- K. Manjunath and V. Venkateswarlu, J. Controlled Release, 2005, 107, 215–228 CrossRef CAS PubMed.
- L. Gastaldi, L. Battaglia, E. Peira, D. Chirio, E. Muntoni, I. Solazzi, M. Gallarate and F. Dosio, Eur. J. Pharm. Biopharm., 2014, 87, 433–444 CrossRef CAS PubMed.
- N. Hussain, V. Jaitley and A. T. Florence, Adv. Drug Delivery Rev., 2001, 50, 107–142 CrossRef CAS PubMed.
- D. Duchene and G. Ponchel, Eur. J. Pharm. Biopharm., 1997, 44, 15–23 CrossRef CAS.
- H. Yuan, J. Chen, Y. Z. Du, F. Q. Hu, S. Zeng and H. L. Zhao, Colloids Surf., B, 2007, 58, 157–164 CrossRef CAS PubMed.
- J. Kreuter, Adv. Drug Delivery Rev., 1991, 7, 71–86 CrossRef CAS.
- H. L. Li, X. B. Zhao, Y. K. Ma, G. X. Zhai, L. B. Li and H. X. Lou, J. Controlled Release, 2009, 133, 238–244 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |