Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel
Elnaz Aliyari,
Mahrouz Alvand and
Farzaneh Shemirani*
School of Chemistry, University College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Iran. E-mail: shemiran@khayam.ut.ac.ir; Fax: +98 21 66405141; Tel: +98 21 61112481
First published on 29th June 2016
Abstract
A new selective and high-capacity magnetic solid phase extraction sorbent was developed for preconcentration of trace amounts of nickel ions prior to their determination by flame atomic absorption spectrometry (FAAS). The sorbent was prepared by immobilization of the dimethylglyoxime ligand (DMG) onto magnetic graphene oxide nanoparticles (MGO) coated with the surface-active ionic liquid-based surfactant, 1-hexadecyl-3-methylimidazolium chloride ([C16MIM][Cl]). The prepared adsorbent (DMG-C16MIM/MGO) was characterized by FESEM, TEM, BET, XRD, VSM and FT-IR spectroscopy. The fabricated adsorbent combines the advantages of the superior adsorption capability of modified graphene oxide and magnetic separability of magnetic nanoparticles to provide high adsorption capacity, and easy isolation from sample solutions. Several important parameters influencing the extraction efficiency, such as pH, amount of adsorbent, extraction time, elution conditions, sample volume, interfering ions and adsorption capacity were studied and optimized. Applying all optimum conditions in the process, a high preconcentration factor of 100, linear range of 0.56–200 μg L−1, detection limit of 0.16 μg L−1, and precision (RSD%, n = 6) of 1.21%, were obtained for nickel. Following modification with 1-hexadecyl-3-methylimidazolium chloride and DMG, the modified adsorbent selectivity toward nickel ions was raised more than nine-fold compared to the unmodified magnetic graphene oxide. The adsorption capacity within a 15 min interaction time based on the Langmuir model was 129.87 and 26.59 mg g−1 for modified and unmodified adsorbents, respectively. The recoveries in the case of real samples varied within the range of 96.8–99.2% confirming good performance of the method in various real samples.
Introduction
Sample pretreatment prior to instrumental analysis is a crucial step in a whole analytical process, especially in the analysis of trace analytes in complex matrices. To date, a variety of methods have been developed for the separation and preconcentration of target compounds from various samples.1,2 Among these methods, magnetic solid phase extraction (MSPE) appears to be one of the most effective selections owing to its ease of automation, high extraction efficiency and rapid phase separation. In MSPE, the magnetic materials and sample solution could be easily separated with an external magnetic field without the need of centrifugation or filtration.3,4According to the nature of this method, choosing an appropriate adsorbent plays an influential role in precision and sensitivity of extraction process. It is obvious that the excellent absorbent materials should have high specific surface area, chemical stability, good sorption properties and a lot of adsorption sites.5 Carbon-based materials have been successfully used as solid adsorbents in SPE due to their large specific surface area and excellent adsorption capacity.6
Graphene oxide (GO) is a flat single layer of sp2- and sp3-hybridized carbon atoms arranged in a hexagonal lattice.7 Biocompatible GO nanosheets contain various functional groups such as epoxy (C–O–C), hydroxyl (OH), and carboxyl (COOH), on both basal planes and edges which lead GO to participate in a wide range of bonding interactions.8 Considering its low manufacturing costs, large specific surface area, long-term stability and the amphiphilic nature, GO is expected to be an attractive material for a wide variety of applications.9 In order to entirely use all the potential advantages of graphene oxide in SPE, fabrication of GO/metal oxide composites is expected to be an effective and practical method. The introduction of superparamagnetic Fe3O4 nanoparticles on GO sheets lead magnetic graphene oxide (MGO) nanocomposite to take the benefits of the magnetic phase separation convenience and graphene oxide nanosheets adsorption capacity and rapid mass transfer which is beneficial for rapid equilibrium, at the same time.10,11 Furthermore, the surface modification of magnetic graphene oxide can lead to selective separation of analytes from samples with complicated matrices.12 Various reagents, including polymers,13 porphyrins14 and ionic liquids,15 have been used to functionalize magnetic graphene oxide nanocomposites to improve their chemical properties.
Ionic liquids (ILs) are a class of organic salts which are composed of organic cations and inorganic or organic anions.16 ILs possess many unique physicochemical properties, such as good stability, low toxicity, negligible vapor pressure and tunable miscibility.17 Thus, they are regarded as promising “green” materials for a number of analytical applications.18–20 Among the varieties of ILs, some ILs with long-chain alkyl groups that self-assemble to form aggregates in aqueous solutions were studied comprehensively because of their inherent amphiphilic nature; these ILs are known as surface-active ionic liquids (SAILs).21,22 Recently, SAILs have emerged as a novel and important class of surfactants. In comparison with conventional surfactants with analogues structures, SAILs-based surfactants, particularly imidazolium-based SAILs have lower critical micelle concentrations (CMCs), much better extraction efficiencies and one can easily modify their micellar solutions by changing the nature of their cation(s) and anion(s).23–26 Moreover, SAILs have been found to exhibit greater surface active properties as compared to conventional surfactants which can be tuned as per the desired application.27 Recently, only few numbers of publications have investigated on application of SAIL-based surfactants in the field of analytical chemistry.28–31 For instance, Cheng et al. have investigated 1-hexadecyl-3-methylimidazolium bromide-coated Fe3O4 magnetic nanoparticles as an adsorbent of mixed hemimicelles solid phase extraction for the preconcentration of chlorophenols from environmental samples,28 Pino et al. have applied the ionic liquid 1-hexadecyl-3-methylimidazolium bromide in a microwave-assisted liquid–liquid extraction system for polycyclic aromatic hydrocarbons (PAHs) in sediments.29 However, the application of this group of ILs in the field of analytical chemistry is still limited.
In the present work, a novel nano-adsorbent with high adsorption capacity was prepared by immobilization dimethylglyoxime ligand onto magnetic graphene oxide nanoparticles coated with SAIL-based surfactant, 1-hexadecyl-3-methylimidazolium chloride. Heavy metals are very toxic potentially and tend to accumulate in different vital organs and be the cause of severe diseases and disorders due to their non-biodegradability and persistence.32 Due to the potential adverse effects of these elements and importance of their determination, nickel was employed as a model compound for a proof-of-concept case to test the feasibility of this method. It is the first time to report the magnetic solid phase extraction of metal ions based on modified surface-active ionic liquid-coated magnetic graphene oxide. Due to the high specific surface area of graphene oxide, the combination of magnetic graphene oxide with hemimicelles/admicelles of SAIL-based surfactant can greatly improve the adsorption capacity of the sorbent. Moreover, the introduction of DMG onto magnetic graphene oxide-ionic liquid hybrid nanocomposites leads to enhance the selectivity and extraction performance of magnetic graphene oxide toward nickel ions from complex matrices. Capability and validity of developed MSPE method was investigated by preconcentration and determination of nickel ions in various real samples. Determination of nickel concentration was carried out by flame atomic absorption spectrometry (FAAS) because of its high specificity, satisfactory sensitivity and low cost.
Experimental
Reagents and solutions
All chemicals used in this study were of analytical reagent grade and were used without further purification. Graphite powders (325 mesh) were purchased from Alfa Aesar (Ward Hill, MA, USA). 1-Hexadecyl-3-methylimidazolium chloride, [C16MIM][Cl] was purchased from Sinopharm Chemical Reagent Corporation (Shanghai, China). Potassium permanganate (KMnO4), sodium nitrate (NaNO3), Fe(NO3)3·9H2O, FeSO4·7H2O, dimethylglyoxim (DMG) and all acids, bases and salts were purchased from Merck (Darmstadt, Germany). A stock standard solution (1000 mg L−1) of Ni(II) was prepared by dissolving an appropriate amount of Ni(NO3)2·6H2O in distilled water with addition of 1.0% HNO3. Working standard solutions of lower concentration were prepared on a daily basis using appropriate dilution. The pH adjustment was performed with HCl and NaOH aqueous solutions.Apparatus
Determination of nickel concentration was carried out by an atomic absorption spectrometer AA-400 (Varian Australia Pty Ltd., Musgrave, Victoria, Australia), equipped with deuterium background correction lamp and air–acetylene flame. The nickel hollow cathode lamp (Varian) was employed as the radiation sources at 232 nm. All measurements were carried out in peak height mode. Fourier transform infrared (FT-IR) spectra were recorded with an Equinox 55 (Bruker Optik GmbH, Ettlingen, Germany) using KBr pellet with the ATR method over the wavelength rang of 400–4000 cm−1. A field emission scanning electron microscope (FESEM) model Sigma (ZEISS, Germany) was employed to characterize magnetic nanoparticles. TEM image were taken out using a transmission electron microscope (ZEISS, EM10C-80KV, Germany). BET analysis was performed on Micromeritics ASAP 2020 surface area and porosity analyzer (Quantachrome, United States). Pore distributions and pore volume were calculated using the adsorption branch of the N2 isotherms based on the BJH model. X-ray powder diffraction (XRD) measurements were performed using a STADI-MP (STOE & Cie, GmbH, Darmstadt, Germany) with monochromatised Cu Kα radiation. Magnetization measurement was performed using a vibrating sample magnetometer (VSM/AGFM, Meghnatis Daghigh Kavir Co., Kashan, Iran). A digital pH-meter (Metrohm, model 781, Herisau, Switzerland), equipped with a glass-combination electrode was used for pH adjustment. Magnetic separation was assisted by a strong neodymium-iron-boron (Nd2Fe12B) magnet (1.31 T).Synthesis of magnetic graphene oxide
GO was prepared from graphite powder according to the modified Hummers method.33 The procedure is briefly described as follows. Graphite powder (1 g) and sodium nitrate (1 g) were added to 23 mL of 98% sulfuric acid in an ice bath and the mixture was stirred under vigorous agitation for about 15 min. Then, followed by slow addition of KMnO4 (3 g) while, the rate of addition was carefully controlled to keep the reaction temperature below 20 °C and stirring was maintained for 2 h. In the subsequent step, the temperature was raised to 35 °C and the mixture was stirred for 1 more h. Afterward, 45 mL doubly distilled water was gradually added and the mixture was kept at 98 °C for 30 min. After cooling down to room temperature, 140 mL of doubly distilled water was poured into the mixture. Then, 12 mL of 30% H2O2 was dripped to reduce the unreacted KMnO4. The resulting mixture was separated by centrifuge, washed repeatedly with 5% HCl and distilled water several times. The obtained graphite oxide was dispersed in water and subsequently sonicated to exfoliate and obtain graphene oxide. Finally, the prepared GO was dried in an oven at 60 °C.Fe3O4-GO was synthesized by the in situ ultrasonic-assisted co-precipitation of iron ions in the alkaline solution in the presence of graphene oxide. The molar ratio of Fe2+ and Fe3+ was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. GO (0.7 g) was sonicated in 200 mL of solution containing 100 mL 0.1 mol L−1 Fe(NO3)3·9H2O and 100 mL 0.05 mol L−1 FeSO4·7H2O at 50 °C for 10 min, under nitrogen atmosphere. Then, 30 mL 8 M ammonia solution was added dropwise to precipitate the iron oxides. The pH of the final mixture controlled in the range of 10–12. The reaction was maintained at 50 °C for 30 min to promote the complete growth of the nanoparticle crystals on the GO. The synthesized MGO was separated with an external magnetic field and washed with deionized water for several times, and then dried at room temperature under vacuum.
2. GO (0.7 g) was sonicated in 200 mL of solution containing 100 mL 0.1 mol L−1 Fe(NO3)3·9H2O and 100 mL 0.05 mol L−1 FeSO4·7H2O at 50 °C for 10 min, under nitrogen atmosphere. Then, 30 mL 8 M ammonia solution was added dropwise to precipitate the iron oxides. The pH of the final mixture controlled in the range of 10–12. The reaction was maintained at 50 °C for 30 min to promote the complete growth of the nanoparticle crystals on the GO. The synthesized MGO was separated with an external magnetic field and washed with deionized water for several times, and then dried at room temperature under vacuum.
Modification of magnetic graphene oxide nanocomposite
Modification of MGO nanocomposite was performed through electrostatic self-assembly technique which is very simple, facile and mild. The assembly of [C16MIM][Cl] and DMG onto magnetic graphene oxide nanocomposite was performed by mixing [C16MIM][Cl] and dimethylglyoxime with MGO in a pH 10 aqueous solution. The synthesis protocol is shown in Scheme 1. The high pH value maximizes the negative surface charge on magnetic graphene oxide, which in turn optimizes the adhesion of the cationic ionic liquid. 1-Hexadecyl-3-methylimidazolium chloride ionic liquid has long alkyl chain which can form hemimicelles/admicelles on the surface of magnetic graphene oxide through both hydrophobic interactions and electrostatic attraction. This configuration has the potential to solubilize organic molecules such as DMG within the structures formed. Therefore, an amount of 1 g of prepared magnetic graphene oxide powder was dispersed in 30 mL distilled water and the pH was raised to 10. Next, 1 g [C16MIM][Cl] was dissolved in 10 mL ethanol/water mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and added into aforementioned mixture. The mixture was sonicated for 30 min to ensure complete assembly of [C16MIM][Cl] on MGO nanocomposite. In the subsequent step, 0.3 g dimethylglyoxime was dissolved in 20 mL of water/ethanol mixture (4
2) and added into aforementioned mixture. The mixture was sonicated for 30 min to ensure complete assembly of [C16MIM][Cl] on MGO nanocomposite. In the subsequent step, 0.3 g dimethylglyoxime was dissolved in 20 mL of water/ethanol mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and gradually added to the mixture and the mixture was sonicated for another 30 min to promote DMG assembly onto the C16MIM/MGO hybrid nanocomposite through hydrophobic interactions. Finally, the modified adsorbent (DMG-C16MIM/MGO) was separated with an external magnetic field and washed three times with distilled water and dried at room temperature.
1) and gradually added to the mixture and the mixture was sonicated for another 30 min to promote DMG assembly onto the C16MIM/MGO hybrid nanocomposite through hydrophobic interactions. Finally, the modified adsorbent (DMG-C16MIM/MGO) was separated with an external magnetic field and washed three times with distilled water and dried at room temperature.
Procedure of magnetic solid phase extraction
The adsorption of nickel ions on DMG-C16MIM/MGO nanocomposite was performed applying a batch technique under ambient conditions. Initially, 10 mg of modified sorbent was added into 50 mL sample solution containing 100 μg L−1 of Ni(II) ions and the pH value of the mixture was adjusted approximately to 8. Then, the mixture was shaken for 15 min to reach adsorption equilibrium. Subsequently, solid mass was magnetically separated from the mixture and the supernatant was poured out. Then, the separated solid was eluted with 3 mL of 1.0 M HNO3 solution to desorbe the target ions. Finally, the analyte concentration in the eluent phase was determined by FAAS.Real sample preparation
In order to assess the capability and validity of the proposed method, the developed procedure was applied to determine the amounts of nickel in various types of real samples. Spinach, cacao powder, tea and cigarette were purchased from local markets in Tehran. 5 g of spinach, 1 g of cacao powder, tea and cigarette were placed separately into an oven and heated at 90 °C for 1 h until a constant weight was achieved. After cooling, 10 mL of concentrated nitric acid was added to each sample then heated on a hot plate to 90 °C until brown fumes ceased to evolve. Following that, 10 mL of 30% hydrogen peroxide were added and heating was maintained until the sample became clear. The digested sample was cooled to ambient temperature, filtered through filter paper and made up to volume in a 100 mL flask. Afterward, determination of nickel ions was done according to the procedure outlined above.Two water samples including sea water (the Caspian sea) and river water (the Babolrud river) were collected. Water samples were filtered using a 0.45 μm pore size membrane filter to remove suspended particular solids the developed method was applied to determine nickel contents. Aliquots of 50 mL of each sample were used for extraction of Ni(II), under optimal conditions of the method.
Results and discussion
Characterization of the adsorbent
The surface morphology of the synthesized sorbent was characterized by SEM and TEM. Fig. 1a and b display the typical FESEM images of modified MGO at low and high magnification. As can be seen, GO have a layered and sheet-like structure with the large surface and wrinkled edge. | ||
| Fig. 1 (a, b) SEM micrographs of modified magnetic graphene oxide hybrid nanocomposite at low and high magnification, (c) TEM image of MGO, (d) nitrogen adsorption–desorption isotherm curve. | ||
Representative transmission electron microscopy image of the MGO-C16MIM-DMG nanocomposite is shown in Fig. 1c. Transparency of graphene oxide sheet in the TEM image confirms that obtained sheets through chemical synthesis from graphite, are almost single or few layers which may provide a higher surface area-to-volume ratio to offer more active sorption sites. Also, Fe3O4 nanoparticles with a mean diameter of approximately 8 nm and spherical morphology were well dispersed on the surface of GO sheets. Fe3O4 NPs can act as spacers between graphene oxide sheets to minimize the possibility of serious agglomeration and restacking of the GO sheets. Moreover, it can be observed that Fe3O4 nanoparticles are strongly deposited on the surface of GO sheets, even after a long time sonication during the preparation of TEM specimen, revealing the strong interaction between Fe3O4 NPs and GO.
Specific surface area is one of the most important characteristics of nanocomposites; indeed, a high effective surface area allows for high adsorption capacity of sorbents. According to the N2 sorption analysis, the BET surface area of MGO-C16MIM-DMG is 146.98 m2 g−1 and BJH desorption cumulative volume of pores is 0.189 cm3 g−1. The isotherm is of type IV which is characteristic of mesoporous material. As can be seen from Fig. 1d, isotherm displays a H3 hysteresis loop, indicating the presence of mesopores. The porous structure of MGO-C16MIM-DMG may be due to the Fe3O4 nanoparticles aggregates and their attachment on GO nanosheets whereas folded GO nanosheets and well distributed Fe3O4 nanoparticles intercalated in GO are responsible for porous structure in the synthesized nanocomposite.34
The FT-IR spectra of GO, MGO and DMG-C16MIM/MGO are shown in Fig. 2a–c. In GO spectrum (Fig. 2a), the adsorption bands at about 1054 and 1216 cm−1 are related to stretching vibration of C–O groups, and a band at 1401 cm−1 represents C–O bending vibration. The absorption band at 1626 cm−1 corresponds with aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. The C
C. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching is located at 1729 cm−1. The presence of a broad and intense peak at about 3433 cm−1 corresponds to stretching vibrations of O–H. These results confirm that a large amount of oxygen functional groups exist on GO nanosheets after the oxidation process. In magnetic graphene oxide spectrum (Fig. 2b), the peaks at 475 and 592 cm−1 relate to Fe–O stretching vibrations prove that Fe3O4 nanoparticles were successfully dispersed on GO sheets. In modified magnetic graphene oxide spectrum (Fig. 2c), the absorbance at 1167 cm−1 can be ascribed to the in plane C–H deformation vibration of imidazolium ring. The absorption bands at about 1464 and 1573 cm−1 are attributable to skeletal vibrations of the imidazolium ring which are related to the imidazolium C–N and C–C bending vibrations, respectively. The absorption band at about 1630 cm−1 corresponds with C
O stretching is located at 1729 cm−1. The presence of a broad and intense peak at about 3433 cm−1 corresponds to stretching vibrations of O–H. These results confirm that a large amount of oxygen functional groups exist on GO nanosheets after the oxidation process. In magnetic graphene oxide spectrum (Fig. 2b), the peaks at 475 and 592 cm−1 relate to Fe–O stretching vibrations prove that Fe3O4 nanoparticles were successfully dispersed on GO sheets. In modified magnetic graphene oxide spectrum (Fig. 2c), the absorbance at 1167 cm−1 can be ascribed to the in plane C–H deformation vibration of imidazolium ring. The absorption bands at about 1464 and 1573 cm−1 are attributable to skeletal vibrations of the imidazolium ring which are related to the imidazolium C–N and C–C bending vibrations, respectively. The absorption band at about 1630 cm−1 corresponds with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bands which are presented in the structure of DMG. The adsorption peaks at 2920 and 2851 cm−1 are related to asymmetric and symmetric stretching vibrations of C–H bands in the long alkyl chains of IL and in the structure of DMG.
N bands which are presented in the structure of DMG. The adsorption peaks at 2920 and 2851 cm−1 are related to asymmetric and symmetric stretching vibrations of C–H bands in the long alkyl chains of IL and in the structure of DMG.
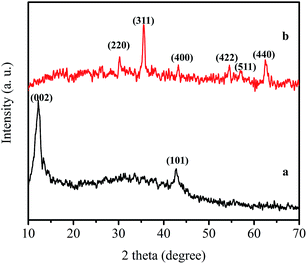
XRD patterns of GO and DMG-C16MIM/MGO were shown in Fig. 3a and b. Fig. 3a shows sharp diffraction peaks at 2θ = 12.26° and 42.75°, corresponding to the (002) and (101) reflection of GO. The XRD pattern of the DMG-C16MIM/MGO nanocomposite (Fig. 3b) indicates six characteristic peaks of Fe3O4 at about 2θ = 30.2, 35.5, 43.1, 54.3, 57.1 and 62.8°, corresponding to their indices (220), (311), (400), (422), (511) and (440), respectively. The positions and relative intensities of all diffraction peaks match well with those from pure magnetite (JCPDS card no. 75-1610). The (002) reflection peak of GO disappeared due to the fact that the GO sheets cannot stack on top of one another to form crystalline structures after being covered with the magnetic nanospheres.
Magnetic properties of MGO before and after self-assembly with IL and DMG were measured using VSM at room temperature and the magnetic hysteresis loops were obtained as an S-like curve (Fig. 4). The magnetic remanence (MR) of MGO and DMG-C16MIM/MGO were 0.0676 and 0.0702 emu g−1, respectively, almost no remaining magnetization when the external magnetic field was eliminated, proving that the synthesized nanocomposites exhibited a superparamagnetic behavior. The saturation magnetization (MS) of the synthesized MGO (Fig. 4a) was found to be 15.25 emu g−1. After grafting 1-hexadecyl-3-methylimidazolium chloride and ligand onto the surface of MGO, the value of MS decreased to 12.96 emu g−1 (Fig. 4b). The decrease of maximal saturation magnetizations of DMG-C16MIM/MGO is caused by the nonmagnetic ionic liquid and DMG layers on the surface of nanocomposite. The results indicate that the magnetic nanocomposites show sensitive response to an external magnetic field.
Effect of pH
Crucial role of the pH of the solution in the SPE procedure is well known and a convenient pH value can improve the adsorption efficiency. It can influence the state of target analyte and the accessibility of binding sites. Hence, the effect of pH on the extraction recovery of target metal ion was evaluated within the range of 4–10. As shown in Fig. 5, extraction recoveries of nickel ions increase with the increasing solution pH and reached to quantitative recovery at pH 8. This may be attributed to the presence of a free lone pair of electrons on the nitrogen atoms in the dimethylglyoxime structure and deprotonated oxygen atoms on the surface of GO. At higher pH value, extraction recoveries start to decrease because of the precipitation of hydroxides species. Based on the above explanations it can be concluded that, pH 8 is the most suitable value for achieving a high efficiency and a good selectivity of nickel on the DMG-C16MIM/MGO nanocomposite. | ||
| Fig. 5 Effect of pH on the recovery of Ni(II). Conditions: amount of metal ions: 100 μg L− 1, sample volume 50 mL, amount of sorbent: 10 mg, contact time: 15 min, eluent: 3 mL of 1 mol L−1 HNO3. | ||
Effect of amount of adsorbent and equilibrium time
Nanosorbents have significantly higher surface area to volume and shorter diffusion route with respect to other sorbents which can cause high extraction efficiency and rapid extraction dynamics. Evaluating of adequate amount of DMG-C16MIM/MGO for quantitative recovery of nickel ions was performed by varying the amount of modified adsorbent within the range of 5 to 25 mg. The obtained results showed that by increasing the adsorbent amounts up to 10 mg due to the increasing of accessible sites, the recovery values increased and then, leveled off. Therefore, 10 mg was selected for the subsequent experiments.In order to acquire an appropriate experimental time, the effect of contact time on extraction efficiency was examined by varying stirring time from 2 to 30 min. The results indicated that satisfactory recoveries could be achieved in 15 min.
Effect of eluent
The nature, concentration and volume of eluting agent have significant effect on the elution process of the retained ions from the solid phase. According to negligible adsorption of target ions in acidic media, desorption of the retained nickel ions may be achieved in a proper acidic solution. Under strong acid conditions, the coordination interaction of chelated ions could be easily disrupted and subsequently target ions are released into the desorption medium. Therefore, a series of selected eluent solutions such as HNO3, HCl and CH3COOH in different concentrations and volumes were tasted. As shown in Table 1, 3 mL of 1.0 mol L−1 HNO3 could fulfil the quantitative recovery of target metal ions. In order to obtain maximum desorption efficiency, effect of desorption time was also investigated in the range of 1–5 min. Based on the obtained results, 2 min was sufficient to achieve quantitative desorption of nickel ions.| Eluent | Volume (mL) | Recovery (%) |
|---|---|---|
| 1.0 mol L−1 CH3COOH | 3 | 44.25 |
| 1.0 mol L−1 HNO3 | 2 | 58.20 |
| 2.0 mol L−1 HNO3 | 2 | 64.16 |
| 1.0 mol L−1 HCl | 3 | 74.61 |
| 1.0 mol L−1 HCl | 2 | 83.27 |
| 2.0 mol L−1 HCl | 2 | 88.91 |
| 1.0 mol L−1 HNO3 | 3 | 99.34 |
Effect of sample volume
Due to the low concentrations of trace metals in real samples, the sample volume is one of the most important parameters in the analysis of real samples which has an influence on the preconcentration factor. Therefore, to assess the possibility of preconcentrating low concentrations of analyte from a large volume, the effect of sample volume on the recovery of nickel ions was investigated. For this purpose, the capability of DMG-C16MIM/MGO to preconcentrate of 5 μg of nickel ions from various volumes across the range of 50–350 mL was studied. The results (Fig. 6) demonstrated that Ni(II) ions were quantitatively recovered when the sample volume was less than 300 mL. As the elution volume was 3 mL, a preconcentration factor of 100 was achieved. | ||
| Fig. 6 Effect of sample volume on the recovery of Ni(II). Conditions: amount of metal ions: 5 μg of Ni(II), amount of sorbent: 10 mg, contact time: 15 min, eluent: 3 mL of 1 mol L−1 HNO3. | ||
Effect of coexisting ions
In order to evaluate whether other ions could interfere during the extraction of the target analyte, the procedure was carried out in presence of cations and anions which often accompany analyte ions in real samples. Interfering ions in different interference-to-analyte ratios were added individually to 50 mL of solutions containing 100 μg L−1 of nickel ions, followed by applying the recommended procedure. The tolerance limit was defined as the maximum concentration of the interfering ion causing a change in the analytical signal no higher than ±5%, when compared with the signal of 100 μg L−1 nickel alone. The obtained tolerance limits are summarized in Table 2. The results imply that all studied interfering ions have no significant effects on the extraction efficiency of target ions which indicate the strong affinity of DMG-C16MIM/MGO toward nickel ions.| Ions | Interference to Ni ratio | Recovery (%) |
|---|---|---|
| Na+ | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98.9 |
| K+ | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100.2 |
| Mg2+ | 1500 | 98.7 |
| Ca2+ | 1200 | 97.1 |
| Cu2+ | 200 | 96.8 |
| Pb2+ | 150 | 96.1 |
| Pd2+ | 300 | 98.3 |
| Co2+ | 400 | 99.2 |
| Zn2+ | 250 | 97.5 |
| NO3− | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98.4 |
| CO32− | 1500 | 96.5 |
| SO42− | 2500 | 98.6 |
Selectivity study
The selectivity factor of Ni(II) with respect to Co, Cd, Mn, Cu and Zn and also the relative selectivity factor based on the data of binary competitive metal ion adsorption were calculated by the following equations:35| D = q/Ce | (1) |
| α = DNi/DM | (2) |
| αr = αm/αn | (3) |
Adsorption isotherms
Equilibrium adsorption isotherms reflect the distribution of the metal ion between the adsorbent and liquid phase when the adsorption process reaches an equilibrium state. In this study, Langmuir and Freundlich isotherms were employed to explain adsorption function of Ni(II) on GO-Fe3O4 and DMG-C16MIM/MGO.36 The Langmuir isotherm theory is based on the hypothesis of monolayer coverage of adsorbate over a homogeneous adsorbent surface with no subsequent interaction among adsorbates. The Freundlich isotherm is derived by assuming a heterogeneous surface with a non-uniform distribution of heat of adsorption over the surface.The linear form of Langmuir and Freundlich equations are expressed as:
| Langmuir isotherm: Ce/qe = Ce/qmax + 1/KLqmax | (4) |
Freundlich isotherm: ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n(ln KF + 1/n(ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce) Ce)
| (5) |
| Sorbents | Langmuir adsorption isotherm parameters | Freundlich adsorption isotherm parameters | |||||
|---|---|---|---|---|---|---|---|
| KL (L mg−1) | qmax (mg g−1) | R2 | RL | KF (mg1−1/n L1/n g−1) | n−1 | R2 | |
| Fe3O4-GO | 0.44 | 26.59 | 0.9983 | 0.31–0.04 | 11.81 | 0.22 | 0.9241 |
| DMG-C16MIM/MGO | 0.15 | 129.87 | 0.9906 | 0.57–0.12 | 22.04 | 0.50 | 0.9683 |
Furthermore, the essential characteristics of the Langmuir isotherm can be described by a separation factor, which is defined by the following equation:
| RL = 1/(1 + KLC0) |
The value of RL represents the shape of the Langmuir isotherm and the nature of the adsorption process, as irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1) or unfavorable (RL > 1).37 From Table 4, the RL values of Langmuir stand between 0 and 1 revealing that the adsorption process of Ni(II) on MGO and modified MGO is favorable.
Reusability investigation
The reuse of solid adsorbents is of great importance for both economic and environmental standpoints. In order to investigate the possibility of regeneration and reusability of the DMG-C16MIM/MGO the effect of eight consecutive separations–desorption cycles were studied under the optimized conditions. The results showed that the amounts of the modified sorbent slightly decreased after five cycles of MSPE procedure. However, no significant decrease in the adsorption capacity and the recoveries of the nickel ions were observed, indicating the good performance and reusability of the prepared DMG-C16MIM/MGO as a sorbent for Ni(II) preconcentration.Analytical performance
Under the optimal conditions, calibration curves was constructed by preconcentrating a series of Ni(II) standards and linearity was maintained in the concentration range of 0.56–200 μg L−1 with a correlation coefficient (R2) of 0.9991. The relative standard deviation (analytical precision) was 1.21% for six replicated measurements of 100 μg L−1 of Ni(II). The limit of detection (LOD) of Ni(II) based on LOD = 3Sb/m definition (where m is the slope of the calibration curve and Sb is the standard deviation for seven blank measurements) was found to be 0.16 μg L−1. The preconcentration factor of 100 was calculated as the ratio of the initial volume of the sample, to the final volume of the eluent.Comparison of the developed method with other methods
Table 5 compares the characteristic data of the present method with those of other reported researches in the literature. It is obvious that DMG-C16MIM/MGO presented the highest adsorption capacity for the analyte ions among all investigated materials that can be attributed to the large specific surface area of MGO and the high affinity of DMG toward nickel ions. The results demonstrated that reported sorbent displayed detection limits, precision and preconcentration factor comparable to or better than other solid phase extraction procedures developed for the determination of Ni(II). Moreover, the higher large linear dynamic range is due to the high effective contact surface area and rapid dynamic extraction of proposed sorbent. Additionally, the magnetic separation greatly improved the separation rate and reduced the analysis time. All the results reveal that the new developed method is a good sample preconcentration technique that can be used for ultra-trace analysis of the target analytes in real samples.| Method | PFa | LODb (μg L−1) | RSDc (%) | Adsorption capacity (mg g−1) | Linear range (μg L−1) | Ref. |
|---|---|---|---|---|---|---|
| a Preconcentration factor.b Limit of detection.c Relative standard deviation. | ||||||
| MGO-DVB-VA-(FAAS) | 40 | 1.37 | 0.82 | — | — | 38 |
| PAN-imp-activated carbon cloth-(FAAS) | 100 | 0.1 | 3.5 | 43.2 | 500–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
39 |
| Walnut SD-SDS-DMG-(FAAS) | 80 | 0.55 | 3.1 | 22.00 | 2–100 | 40 |
| MWCNT-ammonium pyrrolidine dithiocarbamate-(FAAS) | 80 | 0.57 | <5% | 8.90 | — | 41 |
| Triethylenetetramine-MWCNT-(FAAS) | 113 | 2.4 | 2.9 | — | 500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42 |
| DMG-C16MIM/MGO-(FAAS) | 100 | 0.16 | 1.21 | 129.87 | 0.56–200 | This work |
Analysis of real samples
The developed method was used for the determination of trace amounts of Ni(II) in water and acid-digested samples. The obtained results are given in Table 6. To investigate the reliability and accuracy of the method, the real samples were spiked with Ni(II) standards at the concentrations of 0 and 50 μg L−1. For each concentration level, four successive experiments were performed and the mean values were reported, the RSDs were less than 2.4% which show good reproducibility. The recovery of the spiked samples ranged from 96.8% to 99.2%. These results indicate the capability of DMG-C16MIM/MGO for selective preconcentration of trace amounts of nickel in real samples with different matrices.| Sample | Added (μg L−1) | Found | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Sea water (μg L−1) | 0 | ND | 1.7 | — |
| 50 | 48.40 | 1.6 | 96.8 | |
| River water (μg L−1) | 0 | 0.64 | 2.0 | — |
| 50 | 50.26 | 1.8 | 99.2 | |
| Cigarette (μg g−1) | 0 | 2.15 | 1.4 | — |
| 50 | 51.36 | 2.1 | 98.5 | |
| Cacao powder (μg g−1) | 0 | 1.88 | 1.7 | — |
| 50 | 50.83 | 1.8 | 98.0 | |
| Spinach (μg g−1) | 0 | 0.59 | 1.6 | — |
| 50 | 49.92 | 1.9 | 98.7 | |
| Tea (μg g−1) | 0 | 1.12 | 1.5 | — |
| 50 | 49.75 | 2.3 | 97.3 |
Conclusions
In this paper, a novel magnetic solid phase extraction adsorbent, DMG-C16MIM/MGO, was synthesized and used as a selective adsorbent for separation and preconcentration of trace Ni(II) ions from water, cigarette and food samples. The magnetic nano-sorbent is prepared by a self-assembly technique which is very simple, facile and mild. The use of SAIL-based surfactant and DMG as modifier agents of the magnetic graphene oxide lead to producing an adsorbent with the excellent extraction properties of GO, IL, DMG ligand and magnetic nanoparticles. According to the results obtained this method shows a number of advantages, such as low consumption of ionic liquid, shorten extraction time for the adsorption, high selectivity towards Ni(II) over other ions and high adsorption capacity. Comparative studies showed that by modification with SAIL-based surfactant and DMG, modified magnetic graphene oxide adsorption capacity toward nickel ions raised 4.88 times respect to non-modified magnetic graphene oxide. In addition, the magnetic separation greatly improved the separation rate while avoided the time-consuming column passing or filtration operation. Furthermore, satisfactory recoveries can be obtained using lower amount of modified sorbent. The other benefits of the method are its simplicity, ease of operation, good accuracy and precision, low cost, and a good preconcentration factor. Therefore, developed MSPE method has high analytical potential for the preconcentration of trace levels of nickel from various samples with great success.Acknowledgements
Support of this study by The Research Council of University of Tehran through Grant is gratefully acknowledged.References
- A. F. Barbosa, V. M. P. Barbosa, J. Bettini, P. O. Luccas and E. C. Figueiredo, Restricted access carbon nanotubes for direct extraction of cadmium from human serum samples followed by atomic absorption spectrometry analysis, Talanta, 2015, 131, 213 CrossRef CAS PubMed.
- U. Divrikli, A. A. Kartal, M. Soylak and L. Elci, Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations, J. Hazard. Mater., 2007, 145, 459 CrossRef CAS PubMed.
- Q. Wu, G. Zhao, C. Feng, C. Wang and Z. Wang, Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples, J. Chromatogr. A, 2011, 1218, 7936 CrossRef CAS PubMed.
- G. Giakisikli and A. N. Anthemidis, Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review, Anal. Chim. Acta, 2013, 789, 1 CrossRef CAS PubMed.
- A. S. Amin and A. A. Gouda, Utility of solid phase spectrophotometry for the modified determination of trace amounts of cadmium in food samples, Food Chem., 2012, 132, 518 CrossRef CAS PubMed.
- B. T. Zhang, X. Zheng, H. F. Li and J. M. Lin, Application of carbon-based nanomaterials in sample preparation: A review, Anal. Chim. Acta, 2013, 784, 1 CrossRef CAS PubMed.
- Y. Li, J. Qu, F. Gao, S. Lv, L. Shi, C. He and J. Sun, In situ fabrication of Mn3O4 decorated graphene oxide as a synergistic catalyst for degradation of methylene blue, Appl. Catal., B, 2015, 162, 268 CrossRef CAS.
- R. Sitko, B. Zawisza and E. Malicka, Graphene as a new sorbent in analytical chemistry, Trends Anal. Chem., 2013, 51, 33 CrossRef CAS.
- X. Qiu, L. Lu, J. Leng, Y. Yu, W. Wang, M. Jiang and L. Bai, An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine, Food Chem., 2016, 190, 889 CrossRef CAS PubMed.
- Q. Han, Z. Wang, J. Xia, S. Chen, X. Zhang and M. Ding, Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water sample, Talanta, 2012, 101, 388 CrossRef CAS PubMed.
- Q. Wu, C. Feng, C. Wang and Z. Wang, A facile one-pot solvothermal method to produce superparamagnetic graphene–Fe3O4 nanocomposite and its application in the removal of dye from aqueous solution, Colloids Surf., B, 2013, 101, 210 CrossRef CAS PubMed.
- S. Bai and X. Shen, Graphene–inorganic nanocomposites, RSC Adv., 2012, 2, 64 RSC.
- H. Sereshti, S. Samadi, S. Asgari and M. Karimi, Preparation and application of magnetic graphene oxide coated with a modified chitosan pH-sensitive hydrogel: an efficient biocompatible adsorbent for catechin, RSC Adv., 2015, 5, 9396 RSC.
- P. Shi and N. Ye, Investigation of the adsorption mechanism and preconcentration of sulfonamides using a porphyrin-functionalized Fe3O4-graphene oxide nanocomposite, Talanta, 2015, 143, 219 CrossRef CAS PubMed.
- Y. Huang, Y. Wang, Y. Wang, Q. Pan, X. Ding, K. Xu, N. Li and Q. Wen, Ionic liquid-coated Fe3O4/APTES/graphene oxide nanocomposites: Synthesis, characterization and evaluation in protein extraction processes, RSC Adv., 2016, 6, 5718 RSC.
- C. Wua, J. Fana, J. Jianga and J. Wang, pH/temperature dependent selective removal of trace Cr(VI) from aqueous solution by imidazolium ionic liquid functionalized magnetic carbon nanotubes, RSC Adv., 2015, 5, 47165 RSC.
- X. Liu, X. Lu, Y. Huang, C. Liu and S. Zhao, Fe3O4@ ionic liquid@ methyl orange nanoparticles as a novel nano-adsorbent for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples, Talanta, 2014, 119, 341 CrossRef CAS PubMed.
- X. Y. Jia, Y. Han, X. L. Liu, T. C. Duan and H. T. Chen, Dispersive liquid–liquid microextraction combined with flow injection inductively coupled plasma mass spectrometry for simultaneous determination of cadmium, lead and bismuth in water samples, Microchim. Acta, 2010, 171, 49 CrossRef CAS.
- J. F. Liu, J. F. Peng, Y. G. Chi and G. B. Jiang, Determination of formaldehyde in shiitake mushroom by ionic liquid-based liquid-phase microextraction coupled with liquid chromatography, Talanta, 2005, 65, 705 CrossRef CAS PubMed.
- P. Liang and L. Peng, Ionic liquid-modified silica as sorbent for preconcentration of cadmium prior to its determination by flame atomic absorption spectrometry in water samples, Talanta, 2010, 81, 673 CrossRef CAS PubMed.
- B. Dong, N. Li, L. Zheng, L. Yu and T. Inoue, Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution, Langmuir, 2007, 23, 4178 CrossRef CAS PubMed.
- R. Sharma, T. S. Kang and R. K. Mahajan, Complexation of Triblock Reverse Copolymer 10R5 with Surface Active Ionic Liquids in Aqueous Medium: A Physico-chemical Study, RSC Adv., 2015, 5, 16349 RSC.
- V. Pino, C. Yao and J. L. Anderson, Micellization and interfacial behavior of imidazolium-based ionic liquids in organic solvent–water mixtures, J. Colloid Interface Sci., 2009, 333, 548 CrossRef CAS PubMed.
- Q. Zhang, F. Yang, F. Tang, K. Zeng, K. Wu, Q. Cai and S. Yao, Ionic liquid-coated Fe3O4 magnetic nanoparticles as an adsorbent of mixed hemimicelles solid-phase extraction for preconcentration of polycyclic aromatic hydrocarbons in environmental samples, Analyst, 2010, 135, 2426 RSC.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Self-aggregation of ionic liquids: micelle formation in aqueous solution, Green Chem., 2007, 9, 481 RSC.
- T. Singh and A. Kumar, Aggregation behavior of ionic liquids in aqueous solutions: effect of alkyl chain length, cations, and anions, J. Phys. Chem. B, 2007, 111, 7843 CrossRef CAS PubMed.
- P. Bharmoria and A. Kumar, Interactional behaviour of surface active ionic liquids with gelling biopolymer agarose in aqueous medium, RSC Adv., 2013, 3, 19600 RSC.
- Q. Cheng, F. Qu, N. B. Li and H. Q. Luo, Mixed hemimicelles solid-phase extraction of chlorophenols in environmental water samples with 1-hexadecyl-3-methylimidazolium bromide-coated Fe3O4 magnetic nanoparticles with high-performance liquid chromatographic analysis, Anal. Chim. Acta, 2012, 715, 113 CrossRef CAS PubMed.
- V. Pino, J. L. Anderson, J. H. Ayala, V. Gonzalez and A. M. Afonso, The ionic liquid 1-hexadecyl-3-methylimidazolium bromide as novel extracting system for polycyclic aromatic hydrocarbons contained in sediments using focused microwave-assisted extraction, J. Chromatogr. A, 2008, 1182, 145 CrossRef CAS PubMed.
- D. Xiao, D. Yuan, H. He, C. Pham-Huy, H. Dai, C. Wang and C. Zhang, Mixed hemimicelle solid-phase extraction based on magnetic carbon nanotubes and ionic liquids for the determination of flavonoids, Carbon, 2014, 72, 274 CrossRef CAS.
- X. Liu, X. Lu, Y. Huang, C. Liu and S. Zhao, Fe3O4@ ionic liquid@ methyl orange nanoparticles as a novel nano-adsorbent for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples, Talanta, 2014, 119, 341 CrossRef CAS PubMed.
- A. A. Asgharinezhad, N. Jalilian, H. Ebrahimzadeh and Z. Panjali, A simple and fast method based on a new magnetic ion imprinted polymer nanoparticles for the selective extraction of Ni(II) ions in different food samples, RSC Adv., 2015, 5, 45510 RSC.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- W. Baaziz, L. Truong-Phuoc, C. Duong-Viet, G. Melinte, I. Janowska, V. Papaefthimiou, O. Ersen, S. Zafeiratos, D. Begin, S. Begin-Colin and C. Pham-Huu, Few layer graphene decorated with homogeneous magnetic Fe3O4 nanoparticles with tunable covering densities, J. Mater. Chem. A, 2014, 2, 2690 CAS.
- S. Wang and R. Zhang, Selective solid-phase extraction of trace copper ions in aqueous solution with a Cu(II)-imprinted interpenetrating polymer network gel prepared by ionic imprinted polymer (IIP) technique, Microchim. Acta, 2006, 154, 73 CrossRef CAS.
- J. Sun, Q. Liang, Q. Han, X. Zhang and M. Ding, One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples, Talanta, 2015, 132, 557 CrossRef CAS PubMed.
- S. Q. Memon, N. Memon, S. W. Shah, M. Y. Khuhawar and M. I. Bhanger, Sawdust–A green and economical sorbent for the removal of cadmium (II) ions, J. Hazard. Mater., 2007, 139, 116 CrossRef CAS PubMed.
- M. Khan, E. Yilmaz, B. Sevinc, E. Sahmetlioglu, J. Shah, M. R. Jan and M. Soylak, Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions, Talanta, 2016, 146, 130 CrossRef CAS PubMed.
- Z. A. Alothman, E. Yilmaz, M. Habila and M. Soylak, Solid phase extraction of metal ions in environmental samples on 1-(2- pyridylazo)-2-naphthol impregnated activated carbon cloth, Ecotoxicol. Environ. Saf., 2015, 112, 74 CrossRef CAS PubMed.
- M. H. Baki, Farzaneh Shemirani, Surfactant modified walnut sawdust as an alternative green support for efficient preconcentration of nickel ions from different real samples, Anal. Methods, 2013, 5, 3255 RSC.
- M. Tuzen, K. O. Saygi and M. Soylak, Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes, J. Hazard. Mater., 2008, 152, 632 CrossRef CAS PubMed.
- Z. A. Alothman, E. Yilmaz, M. A. Habila, I. H. Alsohaimi, A. M. Aldawsari, N. M. AL-Harbi and M. Soylak, Triethylenetetramine modified multiwalled carbon nanotube for efficient enrichment of Pb(II), Cu(II), Ni(II) and Cd(II) before FAAS detection, RSC Adv., 2015, 5, 106905 RSC.
| This journal is © The Royal Society of Chemistry 2016 |