3D TiO2/ZnO composite nanospheres as an excellent electron transport anode for efficient dye-sensitized solar cells†
Abstract
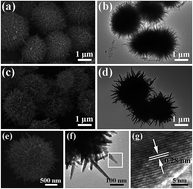
The present study aimed to develop a high electron lifetime (τr) and low transit time (τd) photoanode material for dye sensitized solar cells (DSSCs). An innovative material structure, a TiO2/ZnO composite oxide hierarchical nanosphere, was synthesized by a two-step facile hydrothermal method. This composite oxide comprised 3D urchin-like TiO2 nanospheres and 1D ZnO nanospindles (ULTZ). The ZnO nanospindles were assembled onto the surface of the 3D urchin-like TiO2 in the hydrothermal process second step. A series of ULTZs made with different growth times were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The DSSCs based on the ULTZs exhibited much higher photoelectric properties and the energy conversion efficiency was 8.78%, which indicated a 30% increase in the conversion efficiency compared to those of the P25 electrode (6.79%); the great improvements of photoelectric properties and energy conversion efficiency for ULTZs based DSSCs were mainly attributed to the superior electronic transmission characteristics.

 Please wait while we load your content...
Please wait while we load your content...