The effect of sonication treatment of graphene oxide on the mechanical properties of the assembled films†
Shibing Ye and
Jiachun Feng*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science and Laboratory of Advanced Materials, Fudan University, Shanghai 200433, China. E-mail: jcfeng@fudan.edu.cn; Fax: +86 21 6564 0293; Tel: +86 21 6564 3735
First published on 13th April 2016
Abstract
Graphene oxide films (GOFs) are candidates for structural materials in many applications, but their mechanical properties are frequently divergent, inconsistent, and poorly reproducible. During the fabrication of GOFs, sonication treatment of graphite oxide (GiO) has become a standard step in preparing graphene oxide (GO) solutions prior to assembly. In this work, we systematically studied the effect of sonication treatment of GiO on the mechanical properties of the final GOFs. GOF made from the initial sonication-free GiO solution has large elongation but very low fracture strength and toughness, mainly due to the inhomogeneous structures formed with the incompletely exfoliated GiO flakes. In contrast, a mild sonication within 2 min fully exfoliates the GiO flakes and simultaneously maintains a large amount of large-size GO sheets. The resulting GOF achieves a good balance of both high strength and large elongation, and hence exhibits a superior toughness (1.09 ± 0.14 MJ m−3). However, when further increasing the sonication time to 10, 30, or 60 min, the mechanical properties of GOFs gradually deteriorate, primarily attributed to the significantly reduced GO sizes under intense continuous sonication. Our study provides an insight into the relationship between the sonication time of GiO and the mechanical properties of GOFs, and this knowledge may help to devise better strategies to achieve high-performance GOFs and other GO-based materials.
Introduction
Graphene oxide (GO) has attracted increasing attention from researchers as a two-dimensional (2D) soft amphiphilic material which has a large quantity of oxygen-containing groups on its conjugated planes and along the sheets edges.1–3 This favorable structure and functional groups of GO facilitate its assembly into GO films (GOFs), which has been the most feasible and highly efficient strategy to achieve collective, or enhanced optical, electrical, thermal, and mechanical properties.4 Notably, GOFs play an important role in many technological applications, including separation membranes,5,6 surface coatings,7 transparent and flexible electronics,8 micro- and nanoscale actuators,9 and high-strength fibers.10 Obviously, GOFs require certain mechanical properties to withstand the mechanical loads and harsh environments that arise in commercial applications and retain structural integrity for long-term using. Since the pioneering work of Ruoff et al.11 on the vacuum-filtered GOFs, many researchers have independently prepared GOFs by the same method.12–16 The mechanical properties of these pristine GOFs cover a wide range (modulus: 6–42 GPa, tensile strength: 34–170 MPa, elongation at break: 0.4–2.6%),17–21 which are frequently divergent, inconsistent, and poorly reproducible. The limited studies on the factors driving such individual differences suggest that GOF is a rather complicated system, in which both GO quality and film structure affect their mechanical properties.16,22,23Generally, fabrication of GOFs involves three typical steps: (1) synthesis of starting material, graphite oxide (GiO) derived from graphite, (2) preparation of homogeneous GO solutions, and (3) assembly of GO sheets into free-standing films. It is worth noting that, no matter what form it is stored (powder or solution), sonication treatment of GiO has become a standard step in preparing GO solutions prior to assembly (Table S1†).11,14,15,18,21,24 The use of sonication treatment could decrease the amount of GiO precipitates and fully exfoliate the multilayer GiO flakes into individual GO sheets.25 However, long-time sonication also causes extensive GO sheets fragmentation and defects, which is undesirable from the standpoint of preserving inherent properties in the GO-based materials.26–28 Despite this accepted fact, it appears to be unduly neglected in the fabrication of GOFs. According to the statistical results (Table S1†), the initial GiO mostly underwent long periods of sonication (30–60 min) before assembly into films. Unfortunately, we are unaware of any deliberate works in the literature on the rational control of the parameters of sonication of GiO during the fabrication of GOFs. Therefore, a systematic exploration of the effect of sonication treatment is highly required to provide a reasonable correction for optimizing the mechanical properties of GOFs.
Herein, we address the effect of sonication time on the mechanical properties of GOFs, including fracture strength, elongation at break, and toughness. GOF made from the sonication-free GiO solution shows large elongation but very low fracture strength and toughness, mainly due to the inhomogeneous structures formed with the incompletely exfoliated GiO flakes. In contrast, a mild sonication within 2 min not only fully exfoliates GiO flakes, but also maintains a majority of large-size GO sheets. The resulting GOF achieves a good balance of both high strength and large elongation, and hence exhibits a superior toughness (1.09 ± 0.14 MJ m−3), which is among the highest values of the pristine GOFs fabricated by the same method. However, GOFs' mechanical properties gradually deteriorate with increasing the sonication time, primarily attributed to the significantly reduced GO sizes under intense continuous sonication. For the GiO powders prepared by freeze-drying or vacuum-drying, they have to undergo long periods of sonication (30 or 60 min) to obtain homogeneous dispersion of individual GO sheets. Such long-time sonication breaks GO sheets into small fragments below 1 μm and results in a serious deterioration in the mechanical properties of GOFs. The results in our study may serve as a general guideline for the preparation of GOFs and other GO-based materials.
Experimental sections
Synthesis of GiO
GiO was synthesized by the method proposed by Marcano et al.29 Typically, graphite (5 g, Shengtai graphite Company, China) was dispersed in a mixture of H2SO4 (200 mL) and H3PO4 (40 mL) under stirring with a magnetic stir bar in an ice bath. After stirring for 30 min, KMnO4 (25 g) was slowly added and dissolved within 30 min. The oxidation of graphite was allowed to proceed for another 6 h in a 40 °C-bath. On completing the reaction, the cooling black slurry was poured slowly into cold water (600 mL) under stirring, followed by adding 35% H2O2 (40 mL). After that, the GiO dispersion turned bright yellow, and it was left overnight to allow for complete neutralization of KMnO4. To purify the GiO, the supernatant of the yellow GiO dispersion was decanted, and the GiO precipitate was dispersed in 5 wt% H2SO4 (600 mL), followed by centrifugation at 8000 rpm for 10 min (3×). Then the GiO precipitate was dispersed in deionized water again, followed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min (3×). The obtained GiO slurry was diluted and dialyzed in ample deionized water for 7 days. Large unexfoliated graphite particles that might be present were removed by centrifugation at 4000 rpm for 5 min. The final dialyzed GiO was stored in the form of solutions (∼8 mg mL−1) or powders produced by freeze-drying at −48 °C and vacuum-drying at 60 °C.
000 rpm for 20 min (3×). The obtained GiO slurry was diluted and dialyzed in ample deionized water for 7 days. Large unexfoliated graphite particles that might be present were removed by centrifugation at 4000 rpm for 5 min. The final dialyzed GiO was stored in the form of solutions (∼8 mg mL−1) or powders produced by freeze-drying at −48 °C and vacuum-drying at 60 °C.
Fabrication of GOFs
GiO used in this study comes from the same pot, which ensures the homogeneity and stability of starting material. As illustrated in Fig. 1, GiO was stored in different forms, including as-prepared solution and dried powders. For the GiO solution, it could be directly diluted without sonication to produce aGO(0) solution or sonicated for various time (t min) to produce aGO(t) solutions (KQ100DB ultrasonic cleaner, Kunshan, China, 100 W, 40 kHz). For the freeze-dried and vacuum-dried GiO powders, they need extensive sonication for relatively long time (t min) to obtain homogeneous FDGO(t) and VDGO(t) solutions, respectively (Fig. S1†). GOFs were fabricated by performing vacuum-assisted filtration of above GO solutions (25 mL, 1 mg mL−1) through polycarbonate membranes (diameter 47 mm, pore size 0.2 μm). The resulting GOFs were correspondingly named as aGOF(0), aGOF(t), FDGOF(t), and VDGOF(t). In order to avoid the inconsistency of data due to the atmospheric humidity, all the GOFs were placed in a vacuum chamber at 60 °C for 2 days to prevent excessive exposure to moisture.Characterization and tests
Atomic force microscopy (AFM) images were taken with a AFM (Bruker Multimode V8, USA) with tapping mode. Before measuring, the GO sheets were deposited on a freshly cleaved mica surface by spin coating. X-ray diffraction (XRD) patterns of GOFs were measured by a X-ray diffractometer (PANalytical X'pert PRO, Netherlands) with Cu Kα radiation. Cross-sections of GOFs were obtained by fracturing samples in liquid nitrogen, which were characterized using a field-emission scanning electron microscope (SEM) (Zeiss Ultra 55, German) at an acceleration voltage of 3 kV. Since aGO(0) with large sheet sizes (>10 μm) cannot be completely captured by AFM, SEM was also used to characterize the size dimension of sheets. Samples used for SEM characterizations were prepared by depositing aGO(0) solution on the SiO2/Si wafers by spin coating. In this work, the lateral size of GO sheets was defined as the mean value of their largest and smallest transverse width, referring to the method proposed by Shi et al.20 Mechanical properties of GOFs were measured using a universal testing machine (SANS CMT-6503, Shenzhen, China) fitted with a 50 N load cell. GOFs for tensile testing were cut into rectangular strips with a width of 2 mm and lengths in the range of 25–35 mm by using two combined razor blades. In a typical measurement, the sample strip was fixed on the rubber edge grips with a length of 10 mm between two clamps and tested at a speed of 1 mm min−1. At least five tests were performed for each sample, from which the average values and standard deviations were derived.Results and discussion
Characterization of GO sheets
GiO was produced through an oxidation treatment of graphite, which is commonly applied by the graphene community.29 The as-prepared GiO solution as starting material was stored in a sealed vial, while a portion of GiO solution underwent freeze-drying and vacuum-drying, which was subsequently collected as powders and stored in separate vials. Since GiO powders are metastable materials which is sensitive to the light and air,30 herein all the dried GiO powders were produced no more than one week before use to avoid the possible degradation.Before fabricating GOFs, GO samples treated for various sonication times were firstly prepared and characterized. The aGO(0) referred to the sonication-free GiO solution which was prepared through simple dilution of the as-prepared GiO colloid by shaking. Fig. 2 shows the typical AFM and SEM images of aGO(0) to reveal its morphology and structure. AFM images (Fig. 2a and S2†) show that most of the sheets are ∼1 nm in thickness, indicating that these sonication-free aGO(0) sheets are monolayer. Because of van der Waals interactions between GO sheets, some GO sheets were overlapped on the substrate. It is also worth noting that a portion of aGO(0) sheets have an average thickness of more than 2 nm, corresponding to some incompletely exfoliated layered GiO flakes. As shown in Fig. 2b, SEM image displays large, integrated aGO(0) sheets with lateral sizes around 10 μm without too many fragments. To quantitatively determine sheet size, more than 150 pieces of sheets were counted from the SEM and AFM images. The size distribution histogram of aGO(0) based on the statistical analysis is shown in Fig. 2c. The sizes of aGO(0) sheets have a wide distribution of 5–20 μm, similar to those reported very recently,20 but much larger than those reported previously.15,31,32
 | ||
| Fig. 2 (a) AFM image, (b) SEM image, and (c) the histogram of size distribution of the sonication-free aGO(0). | ||
The as-prepared GiO solution was also sonicated with increasing times of 2, 10, 30, and 60 min to prepare aGO(2), aGO(10), aGO(30), and aGO(60) solutions, respectively. The representative AFM images of these GO sheets and the corresponding size distribution histograms are shown in Fig. 3a and b, respectively. In all cases of the sonicated samples, GO sheets presented on substrates are completely monolayer with a mean thickness of ∼1 nm (Fig. 3a), indicating that the original GiO flakes could be fully exfoliated by short-time sonication. However, AFM images visually demonstrate that sonication tends to damage and fragment GO sheets into smaller pieces. As shown in the size distribution histograms (Fig. 3b), nearly half (47%) of aGO(2) has a sheet size of 1–5 μm, a minority (14%) of aGO(10) has a sheet size of 1–2 μm, and the sheet size almost completely (97–99%) smaller than 1 μm in both aGO(30) and aGO(60). This suggests that more serious fragmentation of GO sheets occurs with increasing the sonication time of GiO, which agrees with the previous study.33
 | ||
| Fig. 3 (a) AFM images and (b) the histograms of size distribution of aGO(2), aGO(10), aGO(30), and aGO(60) sheets. | ||
In many cases, GiO is generally dried to produce powders for easy transportation and storage. When GiO powders are just soaked in water, layer exfoliation proceeds extremely slowly.34 At this point, long period of sonication has to be adopted to accelerate the exfoliation process before filtration assembly. For vacuum-dried GiO powder, sonication for 60 min in water was applied to produce VDGO(60) solution. As comparison, FDGO(60) solution was also prepared by sonicating the freeze-dried GiO powder in water for 60 min. Note that the freeze-dried GiO powder is relatively fluffier than the vacuum-dried counterpart, which makes its dispersion in water more easily.35 Thus the visually homogenous FDGO(30) solution could also be obtained after 30 min sonication. Based on the analysis of the AFM images and histograms of size distribution (Fig. S3†), it is found that the lateral size of the VDGO(60) sheets completely (100%) decreases to <1 μm. In contrast, there are still 4–5% of the FDGO(60) and FDGO(30) sheets having lateral sizes larger than 1 μm.
Structures of GOFs
To investigate the effect of the sonication treatment of GiO on the structures and mechanical properties of the final GOFs, above GO solutions were assembled into free-standing films by the vacuum-filtration method. Typically, GOFs were fabricated by filtering GO solutions with same concentration through the polycarbonate membranes. Each GOF was peeled off from the membrane and further dried in vacuum oven. The thicknesses of all GOFs were within a narrow range of 5–7 μm determined by SEM, which could avoid the variation in mechanical properties caused by film thickness.16,22 Here we take aGOF(0) and aGOF(60) as the representative samples to show the morphology and structure of GOFs. AFM image reveals that the surface of aGOF(0) made from sonication-free aGO(0) sheets is rough with obvious wrinkles, bulges, and agglomerates (Fig. 4a), which may have formed from the sedimentation of the incompletely exfoliated GiO flakes. In contrast, aGOF(60) made from sonicated aGO(60) sheets has quite smooth and dense surface (Fig. 4b), which is consistent with the fact that only stable and agglomerate-free GO solutions can produce uniform and smooth GOFs.14 From AFM characterization, the root-mean-square roughness of aGOF(0) was calculated to be ∼64 nm, higher than that (∼30 nm) of aGOF(60), which further confirms that the surface of the former is more rough than that of the later. The fracture edges of both aGOF(0) and aGOF(60) exhibit a compact, layered structure through the entire cross-section (Fig. 4c and d), which looks similar to the microstructure for those GOFs assembled using the same method.11,20 GOFs including VDGOF(60), FDGOF(30), and FDGOF(60) almost show similar fracture edges (Fig. S4†), indicating that GO sheets with a broad size distribution could be assembled into highly ordered macroscopic structures under vacuum filtration-induced directional flow.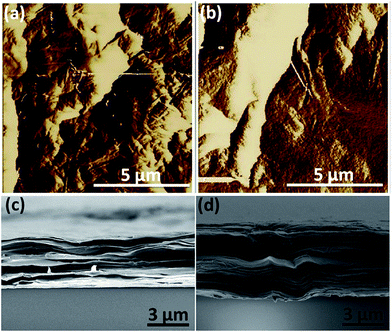 | ||
| Fig. 4 AFM images of the surfaces of (a) aGOF(0) and (b) aGOF(60), and SEM images of the fracture surfaces of (c) aGOF(0) and (d) aGOF(60). | ||
XRD was performed to investigate the internal structures of GOFs. As shown in Fig. 5, the characteristic peak (2θ) of aGOF(0) is measured to 10.38°, corresponding to the d-spacing of 0.852 nm. This value is much larger than that of individual graphene (0.340 nm) because of the presence of oxygenated groups.33 With increasing the sonication time for the GiO, the resulting GOFs exhibit 2θ peaks at 10.19°, 10.13°, 10.12°, and 10.12° for aGOF(2), aGOF(10), aGOF(30), and aGOF(60), respectively. The d-spacings were calculated to be 0.867, 0.872, 0.873, and 0.873 nm correspondingly. Generally, the decreasing GO size during sonication increases the oxidation degree of the smaller GO sheets. Thus, it results in the formation of GOFs with more lattice defects which increase the d-spacing.20,36
Mechanical properties of GOFs
Based on above results, for the GiO stored in any forms, long periods of sonication significantly deteriorates the GO quality and further affects the structures of the final GOFs. Subsequently, we systematically measured the mechanical properties of these GOFs using tensile tests. Each GOF was cut into rectangular strips with a width of 2 mm and lengths in the range of 25 to 35 mm for the tensile tests. As shown Fig. 6a, aGOF(0) has a tensile strength (σ) of 45.8 ± 3.4 MPa, and a ultimate elongation at break (ε) of 3.1 ± 0.3%. When GiO solution was sonicated for 2 min, the obtained aGOF(2) shows a remarkably increased σ of 90.2 ± 13.1 MPa and a slightly decreased ε of 2.4 ± 0.1%. Increasing the sonication time to 10 min results in aGOF(10) with σ = 92.5 ± 7.6 MPa and ε = 2.1 ± 0.3%. If we further increase the sonication time to 30 or 60 min, both σ and ε decreased seriously, which showed σ = 77.5 ± 3.7 MPa and ε = 1.7 ± 0.1% for aGOF(30), and σ = 61.8 ± 9.2 MPa and ε = 1.6 ± 0.1% for aGOF(60).It is interesting to find that aGOF(0) shows the highest elongation at break among all the samples, but a relatively low tensile strength. On the basis of the structure analysis, the sonication-free aGO(0) sheets form rough surfaces with more corrugations and wrinkles in the aGOF(0) (Fig. 4a). These corrugation structures inside the films should influence the fracture strain because the films with more corrugation will be stretched further, like springs before fracture.16,22 Despite the large elongation at break, these wrinkles and agglomerates on the surface also act as surface defects that reduce the fracture strength in some degree. Slight sonication (2 or 10 min) exfoliates GiO flakes well and maintains relatively large GO sizes. GOFs made from large GO sheets always have compact and ordered structure, as less inter-sheet junctions between large GO sheets. It thus appears that aGOF(2) and aGOF(10) have higher fracture strengths (90.2 ± 13.1 and 92.5 ± 7.6 MPa), which were within the normal range of the reported vacuum-filtered GOFs (σ = 62–130 MPa).11,21,24,37 Of course, the smooth surfaces in aGOF(2) and aGOF(10) cannot provide them with additional elongation compared to aGOF(0). Still, their elongation at break (ε = 2.4 and 2.1%) are among the highest reported values and superior to most of the values for GOFs without further modification (σ = 0.4–2.6%) (Table S1†).18,20,21,37 However, long-time sonication (30 or 60 min) breaks the original large GO sheets into rather small pieces, which undoubtedly undermines the mechanical properties of the final GOFs, including both the fracture strengths (77.5 ± 3.7 and 61.8 ± 9.2 MPa) and elongation at break (1.7 ± 0.1 and 1.6 ± 0.1%). This may be because of the more inter-sheet junctions and defects present in the aGOF(30) and aGOF(60) made from very small GO sheets.
As a comparison, the mechanical properties of the GOFs made from VDGO(60), FDGO(30), and FDGO(60) were also measured (Fig. 6b). Among them, the FDGOF(30) has a fairly good mechanical performance (σ = 96.1 ± 5.5 MPa and ε = 2.2 ± 0.3%), which is better than that of aGOF(30) counterpart. This observation may be associated with the relatively large size of FDGO(30) than aGO(30). As revealed in Fig. S3,† there is still 43% of the FDGO(30) sheets larger than 0.5 μm, but this value was decreased to only 14% for aGO(30) after same sonication time (30 min). As we all know, the agglomerate and re-stacking of GO sheets would inevitably happen in the drying process. The energy input of sonication not only breaks GO sheets but also exfoliates the stacked sheets. Ogino et al. found that the repeative freeze-drying process can facilitate the exfoliation of GiO flakes.34 The sonication effect for the dried GiO is more complicated and incomparable with the GiO solution, and the change of sheet size should just be one of the reasons for the better performance of FDGOF(30). For both FDGOF(60) and VDGOF(60), they exhibit obviously deteriorated mechanical properties, similar to the aGOF(60) counterpart, mainly due to the fragmentization of GO sheets after sonication of 60 min.
The volumetric toughnesses of GOFs were calculated and presented in Fig. 6c. aGOF(0) shows a low toughness of 0.71 ± 0.11 MJ m−3 as its fracture strength is rather low. aGOF(2) shows the highest toughness of 1.09 ± 0.14 MJ m−3 because it has good balance of both high elongation and strength. It should be note that this value is higher than most of the reported values.11,15,21,24,38 Our results also demonstrate that the toughness of GOFs gradually decreases with increasing the sonication time of GiO. For both powders and solution, after sonication for 60 min, the resulting GOFs display poor toughness of 0.4–0.6 MJ m−3, which is similar to these GOFs made from GO sheets sonicated for more than 30 min. Accordingly, it appears that sonication of GiO exerts a dominant influence on the mechanical properties of GOFs. This could also explain the variation in the mechanical properties of GOFs among the published papers, and inspires us to optimize the sonication conditions for obtaining GOFs with better performance.
Conclusions
In summary, we present a systematic study on the effect of the sonication treatment of GiO on the mechanical properties of the final GOFs. GOF made from the sonication-free GiO has large elongation but very low fracture strength and toughness, mainly due to the inhomogeneous structures formed by the incompletely exfoliated GiO flakes. In contrast, a mild sonication within 2 min can fully exfoliate GiO flakes and maintain a large amount of large GO sheets. The resulting GOF achieves a good balance of high strength and large elongation, and hence exhibits a superior toughness (1.09 ± 0.14 MJ m−3), which is among the highest values reported in literature for the GOFs prepared by the same method. As the sonication time increases up to 10, 30, and 60 min, the mechanical properties of GOFs gradually deteriorate, which is primarily attributed to the reduced GO sizes caused by sonication. Our results demonstrate that sonication of GiO is an important contributor to the variation in the mechanical properties of GOFs. With this concept in mind, it is therefore worthwhile for researchers to control and optimize the sonication time of GiO prior to preparation of GO-based materials.Acknowledgements
The authors thank Zhouyue Lei for the help in the AFM measurements and Prof. Peiyi Wu for helpful suggestions. This work was financially supported by the Natural Science Foundation of China (51373042) and the Science and Technology Planning Project of Guangdong Province of China (2014B090901009).Notes and references
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed.
- F. Kim, L. J. Cote and J. Huang, Adv. Mater., 2010, 22, 1954–1958 CrossRef CAS PubMed.
- H.-P. Cong, J.-F. Chen and S.-H. Yu, Chem. Soc. Rev., 2014, 43, 7295–7325 RSC.
- L. Huang, Y. Li, Q. Zhou, W. Yuan and G. Shi, Adv. Mater., 2015, 27, 3797–3802 CrossRef CAS PubMed.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- W. Choi, J. Choi, J. Bang and J.-H. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 12510–12519 CAS.
- Z. Wei, D. Wang, S. Kim, S.-Y. Kim, Y. Hu, M. K. Yakes, A. R. Laracuente, Z. Dai, S. R. Marder, C. Berger, W. P. King, W. A. de Heer, P. E. Sheehan and E. Riedo, Science, 2010, 328, 1373–1376 CrossRef CAS PubMed.
- D.-D. Han, Y.-L. Zhang, Y. Liu, Y.-Q. Liu, H.-B. Jiang, B. Han, X.-Y. Fu, H. Ding, H.-L. Xu and H.-B. Sun, Adv. Funct. Mater., 2015, 25, 4548–4557 CrossRef CAS.
- R. Cruz-Silva, A. Morelos-Gomez, H. I. Kim, H. K. Jang, F. Tristan, S. Vega-Diaz, L. P. Rajukumar, A. L. Elias, N. Perea-Lopez, J. Suhr, M. Endo and M. Terrones, ACS Nano, 2014, 8, 5959–5967 CrossRef CAS PubMed.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- Z. An, O. C. Compton, K. W. Putz, L. C. Brinson and S. T. Nguyen, Adv. Mater., 2011, 23, 3842–3846 CAS.
- S. Park, K. S. Lee, G. Bozoklu, W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- H. Chen, M. B. Muller, K. J. Gilmore, G. G. Wallace and D. Li, Adv. Mater., 2008, 20, 3557–3561 CrossRef CAS.
- Y. Tian, Y. W. Cao, Y. Wang, W. L. Yang and J. C. Feng, Adv. Mater., 2013, 25, 2980–2983 CrossRef CAS PubMed.
- T. Gong, D. V. Lam, R. Liu, S. Won, Y. Hwangbo, S. Kwon, J. Kim, K. Sun, J.-H. Kim, S.-M. Lee and C. Lee, Adv. Funct. Mater., 2015, 25, 3756–3763 CrossRef CAS.
- S. Park, D. A. Dikin, S. T. Nguyen and R. S. Ruoff, J. Phys. Chem. C, 2009, 113, 15801–15804 CAS.
- K. W. Putz, O. C. Compton, M. J. Palmeri, S. T. Nguyen and L. C. Brinson, Adv. Funct. Mater., 2010, 20, 3322–3329 CrossRef CAS.
- C.-N. Yeh, K. Raidongia, J. Shao, Q.-H. Yang and J. Huang, Nat. Chem., 2015, 7, 166–170 CrossRef CAS PubMed.
- J. Chen, Y. Li, L. Huang, N. Jia, C. Li and G. Shi, Adv. Mater., 2015, 27, 3654–3660 CrossRef CAS PubMed.
- Y. Wu, R. Cao, L. Ji, W. Huang, X. Yang and Y. Tu, RSC Adv., 2015, 5, 28085–28091 RSC.
- S. Ye, B. Chen and J. Feng, Sci. Rep., 2015, 5, 13102 CrossRef CAS PubMed.
- X. Lin, X. Shen, Q. Zheng, N. Yousefi, L. Ye, Y.-W. Mai and J.-K. Kim, ACS Nano, 2012, 6, 10708–10719 CrossRef CAS PubMed.
- K. Hu, D. D. Kulkarni, I. Choi and V. V. Tsukruk, Prog. Polym. Sci., 2014, 39, 1934–1972 CrossRef CAS.
- S. Pan and I. A. Aksay, ACS Nano, 2011, 5, 4073–4083 CrossRef CAS PubMed.
- Q. F. Cheng, M. X. Wu, M. Z. Li, L. Jiang and Z. Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 3750–3755 CrossRef CAS PubMed.
- Y. Li, R. Umer, Y. A. Samad, L. Zheng and K. Liao, Carbon, 2013, 55, 321–327 CrossRef CAS.
- M. Bracamonte, G. Lacconi, S. Urreta and L. Foa Torres, J. Phys. Chem. C, 2014, 118, 15455–15459 CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Small, 2015, 11, 1266–1272 CrossRef CAS PubMed.
- J. Zhu, H. N. Zhang and N. A. Kotov, ACS Nano, 2013, 7, 4818–4829 CrossRef CAS PubMed.
- Y. Wang, Z. Shi, J. Yu, L. Chen, J. Zhu and Z. Hu, Carbon, 2012, 50, 5525–5536 CrossRef CAS.
- X. Qi, T. Zhou, S. Deng, G. Zong, X. Yao and Q. Fu, J. Mater. Sci., 2014, 49, 1785–1793 CrossRef CAS.
- I. Ogino, Y. Yokoyama, S. Iwamura and S. R. Mukai, Chem. Mater., 2014, 26, 3334–3339 CrossRef CAS.
- Y. Cao, J. Feng and P. Wu, Carbon, 2010, 48, 3834–3839 CrossRef CAS.
- J. Zhao, S. Pei, W. Ren, L. Gao and H.-M. Cheng, ACS Nano, 2010, 4, 5245–5252 CrossRef CAS PubMed.
- Y. Gao, L. Q. Liu, S. Z. Zu, K. Peng, D. Zhou, B. H. Han and Z. Zhang, ACS Nano, 2011, 5, 2134–2141 CrossRef CAS PubMed.
- K. S. Hu, L. S. Tolentino, D. D. Kulkarni, C. H. Ye, S. Kumar and V. V. Tsukruk, Angew. Chem., Int. Ed., 2013, 52, 13784–13788 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Digital pictures of the dispersion of GiO, AFM images of GO sheets, SEM images of GO films, and the statistical results of reported values. See DOI: 10.1039/c6ra03996k |
| This journal is © The Royal Society of Chemistry 2016 |



