Ultrafast pyro-synthesis of NiFe2O4 nanoparticles within a full carbon network as a high-rate and cycle-stable anode material for lithium ion batteries
Abstract
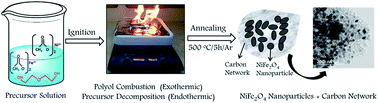
NiFe2O4 nanoparticles fully anchored within a carbon network were prepared via a facile pyro-synthesis method without using any conventional carbon sources. The surface morphology was investigated using field-emission scanning electron microscopy, which confirmed the full anchoring of NiFe2O4 nanoparticles within a carbon network. The primary particle size of NiFe2O4 is in the range of 50–100 nm. The influence of the carbon network on the electrochemical performance of the NiFe2O4/C nanocomposite was investigated. The electrochemical results showed that the NiFe2O4/C anode delivered a reversible capacity of 381.8 mA h g−1 after 100 cycles at a constant current rate of 1.0C, and when the current rate is increased to a high current rate of 5.0C, a reversible capacity of 263.7 mA h g−1 is retained. The obtained charge capacity at high current rates is better than the reported values for NiFe2O4 nanoparticles. The enhanced electrochemical performance can be mainly ascribed to the high electrical conductivity of the electrode, the short diffusion path for Li+ ion transportation in the active material and synergistic effects between the NiFe2O4 nanoparticles and carbon network, which buffers the volume changes and prevents aggregation of NiFe2O4 nanoparticles during cycling.

 Please wait while we load your content...
Please wait while we load your content...