Evolution of iron species for promoting the catalytic performance of FeZSM-5 in phenol oxidation†
Lei Luoa,
Chengyi Daia,
Anfeng Zhanga,
Junhu Wangb,
Chunshan Song*ac and
Xinwen Guo*a
aState Key Laboratory of Fine Chemicals, PSU-DUT Joint Center for Energy Research, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, P. R. China
bMossbauer Effect Data Center, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China
cEMS Energy Institute, PSU-DUT Joint Center for Energy Research and Department of Energy & Mineral Engineering, Pennsylvania State University, University Park, Pennsylvania 16802, USA
First published on 24th March 2016
Abstract
Facile modification of FeZSM-5 utilizing an organic directing agent (ODA) for iron species evolution toward better Fenton activity was developed. Pristine FeZSM-5, prepared hydrothermally using tetrapropylammonium bromide as the ODA, was thermally treated in nitrogen or air atmosphere for comparison. The as-prepared FeZSM-5 were evaluated by XRD, SEM, TGA, Ar physical adsorption, H2-TPR, UV-Vis, XPS and Mössbauer spectra, which confirmed well-dispersed iron species and partial transformation of framework iron to non-framework iron and Fe(III) to Fe(II) during thermal treatment in N2. The phenol oxidation reaction was applied as a probe reaction and phenol conversion can be improved from 44% to 90% within 60 minutes. By thermal treatment in N2 atmosphere, the ODA plays an important role in modifying non-framework iron species from Fe(III) to Fe(II) ions which is desirable for improving catalytic activity in Fenton-like systems. The strategy utilizing the thermal reduction effect of the ODA on iron species is a reliable method for preparing well-dispersed and valence tunable iron oxide nanoparticles encapsulated in ZSM-5.
1 Introduction
Advanced oxidation processes (AOPs), using hydroxyl radicals (·OH), are one of the most effective methods for the destruction of organic pollutants in wastewater, and so have drawn much attention in an extensive range of studies.1–3 Compared with the conventional homogeneous Fenton oxidation system, the activity and stability of heterogeneous catalysts under near neutral conditions are problems that need to be solved.4–7 Regeneration of Fe(II) species is the rate-limiting step in catalytic cycling4,8 and strategies which introduce oxidation–reduction pairs to lower the energy barrier have been proposed.9–11 Though various zeolites have been used due to their acidity and hydrothermal stability,12,13 tuning the chemical state of iron species in zeolite-based catalysts without affecting its dispersion is still a problem for their application.14,15 Therefore, finding a way to optimize the physicochemical state of iron for preventing agglomeration is of great importance.Up to now, many kinds of heterogeneous catalysts have been investigated in the effluent treatment.3,4,16 Metals and metal oxides are the most direct and simplest multiphase catalysts, but they suffer from poor metal dispersion14 and catalyst recovery problem.17 Host–guest materials, like yolk–shell materials,18–20 iron-supported catalyst,11,21–23 iron-exchanged catalyst,13,24 etc., have their special advantages in developing the synergetic effect between host and guest. However, iron-supported catalyst, prepared from impregnation, suffers from metal oxide agglomeration on ZSM-5 external surface14 or from the lower synergetic effect of ordered mesoporous materials25 because of its lower adjustable acidity and hydrothermal stability. In the case of iron-exchanged catalyst, the amount of iron loading greatly depends on the Si/Al ratio of zeolite and low iron content is often obtained. Fortunately, FeZSM-5 obtained from hydrothermal synthesis can not only ensure the great dispersion of iron species but also maintain appropriate iron content. However, both framework iron and non-framework iron species of conventional FeZSM-5 have no satisfactory activity because higher Fe(III) species and lower Fe(II) species exist, which is not desirable for Fenton-like system.8,20,26 Therefore, the direction for modification of FeZSM-5 is improve the content of Fe(II) species.
Reduction of Fe(III) using the thermal reduction effect of organics is a significant method to improve Fe(II) content. By H2 temperature programmed reduction, Fe3O4 can be prepared from Fe2O3, but those Fe3O4 nanoparticles are easy to be oxidized in air. In contrast, organics such as ethylene glycol, polyethylene glycol, and polyvinyl pyrrole, play a very important role in reducing Fe3+ which is similar to H2 in preparing Fe3O4 nanoparticles from Fe(III) salt.27 More importantly, those organics can also stabilize the Fe3O4 from oxidation.27 According to this idea, our previous work15 focused on introducing polyethyleneimine (PEI) to optimize the physicochemical properties of iron oxides. While in order to simplify the preparation process, a more facile strategy was developed in the present work.
Herein, a facile strategy to preparing FeZSM-5 with improved Fenton activity has been developed which involves the use of organic direction agents (ODAs) to reduce non-framework iron species. Phenol oxidation reaction is applied to evaluate the catalytic activity and stability. The structure–activity relationship was also delineated based on the analytical characterization.
2 Experimental
2.1 Chemicals and materials
The chemicals used include silica sol (30 wt%), ethylamine (EA, 65 wt%), tetrapropylammonium bromide (TPABr), tetraethylorthosilicate (TEOS), tetrapropylammonium hydroxide (TPAOH), ferric chloride (FeCl3·6H2O), H2O2, and phenol. All above materials were purchased in reagent grade and used without further treatment.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 TPABr
0.15 TPABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EA
1 EA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 H2O. The active seeding gel was prepared from a gel with a molar ratio of 1 TEOS
17 H2O. The active seeding gel was prepared from a gel with a molar ratio of 1 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.36 TPAOH
0.36 TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 H2O by a conventional hydrothermal synthesis route.11 Typically, a mixture which consists of 50 g of TEOS, 70 g of TPAOH (25 wt%), and 30 g distilled water was stirred at 308 K and maintain for 5 h which followed by increasing temperature to 353 K and crystallize for another 72 h. After cooling down, the obtained seeding gel was directly used for the synthesis of FeZSM-5 zeolites without any treatment. The synthesis of FeZSM-5 was conducted by using the following procedure: first, 13.34 g of TPABr was dissolved with 66.67 g of silica solution and afterwards with a solution of 22.26 g of EA (65 wt%). The mixture was aged at room temperature for 30 min under mechanical stirring followed by addition of a solution of FeCl3·6H2O and 45 g of H2O. 0.21 g as-prepared active seeding gel was then added into the above FeZSM-5 precursor and then introduced to the stainless steel autoclave (200 ml, filled volume 150 ml) with hydrothermal treatment at 443 K for 72 h. The resulting solid was filtered, washed, and dried overnight at 373 K. The as-synthesized zeolite was calcined in nitrogen or air atmosphere at 873 K for 4 h. The resulting sample was denoted as NX or AX, where N and A represent that the calcination atmosphere is nitrogen and air, respectively; X represents the Si/Fe molar ratio of FeZSM-5.
19 H2O by a conventional hydrothermal synthesis route.11 Typically, a mixture which consists of 50 g of TEOS, 70 g of TPAOH (25 wt%), and 30 g distilled water was stirred at 308 K and maintain for 5 h which followed by increasing temperature to 353 K and crystallize for another 72 h. After cooling down, the obtained seeding gel was directly used for the synthesis of FeZSM-5 zeolites without any treatment. The synthesis of FeZSM-5 was conducted by using the following procedure: first, 13.34 g of TPABr was dissolved with 66.67 g of silica solution and afterwards with a solution of 22.26 g of EA (65 wt%). The mixture was aged at room temperature for 30 min under mechanical stirring followed by addition of a solution of FeCl3·6H2O and 45 g of H2O. 0.21 g as-prepared active seeding gel was then added into the above FeZSM-5 precursor and then introduced to the stainless steel autoclave (200 ml, filled volume 150 ml) with hydrothermal treatment at 443 K for 72 h. The resulting solid was filtered, washed, and dried overnight at 373 K. The as-synthesized zeolite was calcined in nitrogen or air atmosphere at 873 K for 4 h. The resulting sample was denoted as NX or AX, where N and A represent that the calcination atmosphere is nitrogen and air, respectively; X represents the Si/Fe molar ratio of FeZSM-5.2.2 Catalytic testing
All catalyst evaluations were conducted in a 100 ml round-bottom flask equipped with a magnetic stirring bar. In a typical procedure, 20 mg catalyst, 50 ml phenol solution (1 g L−1), and 10.76 ml H2O2 solution (0.69 M) were added together into the flask followed by maintaining at 50 °C for the appropriate time. The products were analyzed by liquid chromatography. The molar ratio of H2O2/phenol was 14. Phenol oxidation reaction was chosen to evaluate the catalytic activity of the as-prepared catalyst. In a typical evaluation, 50 mg of the as-prepared catalyst, 10.76 ml of H2O2 solution (0.77 M), and 50 ml of phenol solution (1 g L−1) were added together into a 100 ml round-bottom flask and stirred at 65 °C water bath. The product was sampled every other 15 minutes and analyzed by liquid chromatography (Agent 1200 series) for calculating the phenol conversion.2.3 Characterization
The crystalline structures of the as-prepared catalyst were determined by a Rigaku Smartlab diffractometer with a nickel-filtered Cu Kα X-ray source. The scattering angel was between 5° and 80° at a scanning rate of 0.02°. Morphological properties were obtained on a Hitachi 5-5500 instrument. Prior to the measurement, the samples were sputtered with a thin film of gold. The textual properties including the surface area, pore volume and pore size distribution were measured using a Quantachrome autosorb-iQ2 gas adsorption analyzer with argon at 87 K. Prior to the measurement, the calcined samples were degassed in vacuum at 573 K for 8 h. The total surface area (SBET) was measured by the Brunauer–Emmett–Teller (BET) method. Microporosity and mesoporosity was determined by the t-plot method. H2-TPR measurements were carried out using ChemBET Pulsar TPR/TPD equipment (Quantachrome, USA). The iron content of the as-prepared samples was measured by ICP-OES after digestion with the mixture of HCl, HNO3, HF, HClO4. Ultraviolet-visible diffuse reflectance (UV-Vis) spectra were acquired on a Jasco UV-550 spectrometer, and pure BaSO4 was used as reference. Deconvolution of the UV-Vis spectra into individual iron species' bands was conducted according to the procedure detailed in literature.28 Thermal gravimetric analysis (TGA) was performed on a SDT Q600 (TA Instruments, U.S.A.) with a heating rate of 10 °C min−1 in air or N2 atmosphere. X-ray photoelectron spectra (XPS) were obtained with a Thermo VG ESCALAB250 instrument using Al Kα radiation. The 57Fe Mossbauer spectra were recorded using a Topologic 500 A spectrometer at room temperature with a proportional counter.3 Results and discussion
3.1 Material synthesis and characterization
The XRD patterns of FeZSM-5 series with different Si/Fe ratio and different thermal treatment atmosphere are shown in Fig. 1. Five characteristic diffraction peaks at 7.8°, 8.8°, 23.0°, 23.9° and 24.4° clearly exhibit pure MFI topology of FeZSM-5. No diffraction peaks associated with iron phase were detected, indicating the high dispersion of iron species. Fig. 2 shows the SEM images of the six catalysts with the inset showing their particle size distribution. All six catalysts have a uniform particle size distribution around 700 nm.Fig. 3 shows the Ar adsorption–desorption isotherms of FeZSM-5 at 87 K with the pore size distribution presented in Fig. 4. The adsorption branch of the six samples shows typical type-I adsorption line which indicates their microporosity. Typically, pore distribution at around 0.54 nm represents the oval-shaped and Z-shaped channel, and pore distribution at around 0.9 nm represents the channel intersection.29 The three samples calcined in N2 atmosphere all present a relatively lower pore distribution compared with samples calcined in air which definitely indicate the existence of something blocking their channel intersection. At the same time, total pore volume (Vpore) (shown in Table 1) present the same principle and a relatively smaller pore volume exists in samples calcined in N2. The substance which exists in the intersection is either coke-like species30 or iron species. According to TG curves of sample N40 and A40 (shown in Fig. S1†), weight loss above 600 °C is approximately 0.5 wt% indicating that the organic directing agents of those samples have almost been removed meaning and merely have residual of coke-like species, from which we infer the existence of iron species in channel intersection for three samples calcined in N2.
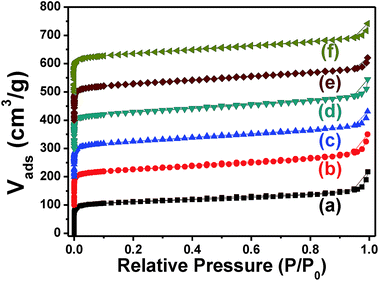 | ||
| Fig. 3 Ar adsorption and desorption isotherms at 87 K (a) N40, (b) A40, (c) N80, (d) A80, (e) N200, (f) A200. The isotherms of each sample are displaced vertically by 100 cm3 g−1. | ||
| Sample code | Smicroa [m2 g−1] | Smesoa [m2 g−1] | SBETb [m2 g−1] | Vmicroc [cm3 g−1] | Vpored [cm3 g−1] |
|---|---|---|---|---|---|
| a t-Plot method.b BET method.c NLDFT method.d p/p0 = 0.99. | |||||
| N40 | 313 | 61 | 374 | 0.182 | 0.28 |
| A40 | 319 | 98 | 417 | 0.197 | 0.32 |
| N80 | 307 | 104 | 411 | 0.193 | 0.29 |
| A80 | 318 | 104 | 422 | 0.199 | 0.31 |
| N200 | 311 | 112 | 423 | 0.199 | 0.28 |
| A200 | 341 | 107 | 448 | 0.213 | 0.31 |
Fig. 5 shows the TGA and DTG curves of the uncalcined samples in N2 and air atmosphere. Weight loss below 300 °C can be attributed to adsorbed water and crystal water, and above 300 °C attributed to the decomposition of TPA+. It can be seen that samples with the same Si/Fe ratio do have a difference when hydrothermal treatment was conducted in different atmosphere. The temperature for decomposition of TPA+ for samples calcined in air is slightly lower than those in N2 which is corresponding with the ref. 31. Owing to the existence of O2 under air atmosphere, the oxidation of TPA+ would release energy. On the contrary, without the help of O2, the decomposition of TPA+ in N2 is merely a pyrolysis process.30,31
 | ||
| Fig. 5 TG curves of uncalcined FeZSM-5 with different Si/Fe ratio. (a) Si/Fe = 40, (b) Si/Fe = 80, (c) Si/Fe = 200. Measure condition: air or N2 atmosphere, 10 °C min−1. | ||
UV-Vis spectra between 190–800 nm were conducted to investigate the nature of Fe(III) species of FeZSM-5,28 and importantly that Fe(II) species do not contribute to the UV-Vis spectra absorbance because the absorbance of Fe(II) species falls in the near infrared range around 1000 nm.32 Fig. 6 shows the UV-Vis spectra of the as-prepared catalysts. For Fe3+ sites, two charge-transfer (CT) bands associated to t1 → t1 and t1 → e transitions are expected.32 Bands at 215 nm and 241 nm can be attributed to framework iron species,33 and a band at 278 nm is attributed Fe3+ species in octahedral sites. Octahedral Fe3+ ions in small oligo nuclear clusters give rise to broad bands between 300 and 450 nm and bands above 450 nm are characteristic for large Fe2O3 particle.28,33 All six samples contain both framework and non-framework iron species but with different contents. According to the ICP-OES analysis (Table 3), samples with lower Si/Fe ratio showed higher iron contents, so it is reasonable that samples with lower Si/Fe ratio show higher intensity in UV-Vis spectra. Importantly, in the case of samples with the same iron contents, for N40 and A40 instance, compared with A40 which was treated under air atmosphere, sample N40 shows weaker absorbance which can be attributed to that sample N40 contained some of Fe(II) ions. Therefore, it can be concluded that the decomposition of TPA+ under N2 result in the generation of Fe(II) species in sample N40. On the other hand, from the deconvolution of N40 and A40 (shown in Table 2), there is very limited change in composition of Fe(III). So there exists such a transformation of iron species to maintain the Fe(III) composition unchanged: framework iron → non-framework iron → Fe(II). And the content of non-framework iron is much higher for samples calcined in N2 than in air.
To investigate the reducibility of the materials, H2-TPR experiments were conducted, as shown in Fig. 7. For all six samples, the three H2 consumption peaks (α1, α2, α3) are attributed to Fe3+ and Fe2O3 to Fe(3−δ)+ (α1), with intermediate valence as that in Fe3O4, and then further reduction to Fe2+ (α2). At higher temperature, the peaks represent partial reduction of Fe2+ to Fe0 (α3). All the above-mentioned iron species in FeZSM-5 that could be reduced by H2 is the non-framework iron species, and higher temperature leads to more framework iron's transformation to non-framework species. According to the H2-TPR profiles of the catalysts, H2 consumption of samples treated in N2 is apparently higher than samples treated in air indicating higher amount of non-framework iron species, in accordance with the UV-Vis analysis. On the other hand, the reduction temperature of sample N40 slightly shifts to lower temperature which shows that iron species in sample N40 are easier to be reduced. This indicates that non-framework iron species in samples treated in N2 atmosphere have a better dispersion.
Fig. 8 shows the Fe 2p XPS spectra of N40 and A40. From the deconvolution of the XPS spectra for Fe 2p3/2, the peak centered at 709.8 eV is attributed to Fe2+.34 For the peak centered at 714.0 eV, it is attributed to the framework iron with the Fe–O–Fe bonds that make the peaks shifting to higher binding energy. In the case of sample A40, as some framework iron species were converted to non-framework species after thermal treatment, the binding energy correspondingly shifts to the higher value centered at 712.4 eV.
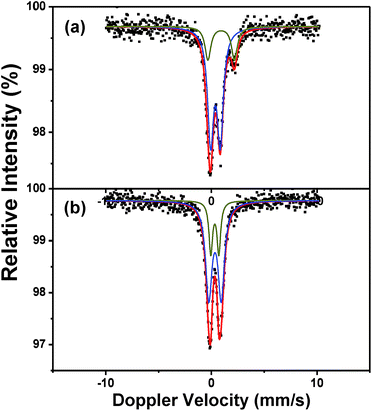
To further investigate the nature of iron species, 57Fe Mössbauer spectroscopic characterization was conducted. Fig. 9 shows the Mössbauer spectra of N40 and A40 at room temperature, with the hyperfine interaction parameters summarized in Table 4. In general, the nanoparticle size of iron species can be discussed by the peak shape including doublet and sextet.35,36 Nanoparticles with high dispersion would present super-paramagnetic phenomenon therefore the peaks would be doublet. From Fig. 9, doublets are observed both N40 and A40 which imply well-dispersion of iron species. Both of the Mössbauer spectra of samples N40 and A40 could be fitted well by two doublets. In the case of N40, the doublet with IS = 0.38 mm s−1 and QS = 0.92 mm s−1 could be attributed to Fe(III) species, and doublet with IS = 0.92 mm s−1 and QS = 2.51 mm s−1 could attributed to Fe(II) species.37–39 In contrast, according to the 57Fe Mössbauer parameters, both of the doublets of A40 only could be attributed to Fe(III) species which is consistent with UV-Vis and XPS analysis.
| Sample | Oxidation state | Sub-spectrum | IS (mm s−1) | QS (mm s−1) | RI (%) |
|---|---|---|---|---|---|
| N40 | Fe(III) | Doublet | 0.38 | 0.92 | 77.3 |
| Fe(II) | Doublet | 0.92 | 2.51 | 22.7 | |
| A40 | Fe(III) | Doublet | 0.32 | 0.76 | 23.8 |
| Fe(III) | Doublet | 0.34 | 1.18 | 76.2 |
3.2 Catalytic performance
As Fenton-like system is very sensitive to the iron chemical state, phenol oxidation reaction is applied as a probe reaction to evaluate the as-prepared FeZSM-5. Fig. 10 and 11 show the phenol and H2O2 conversion, respectively, in the presence of series of FeZSM-5 samples. According to the initial conversion of phenol and H2O2, the samples with higher iron contents exhibit higher activity, and, importantly, samples calcined in N2 exhibit higher catalytic activity than that in air. The obtained products for phenol oxidation is mainly CO2 and H2O, and the intermediate products including catechol, hydroquinone, o-benzoquinone, p-benzoquinone, oxalic acid, acetic acid. | ||
| Fig. 10 Phenol conversion in the presence of (a) N40, (b) A40, (c) N80, (d) A80, (e) N200, (f) A200. | ||
3.3 Discussion
Hydroxyl radical (·OH), produced from H2O2 in chain reaction initiated by Fe2+ (Table 5), is widely accepted as a strong oxidant for its high redox potential in Fenton system. Higher amount of Fe2+ as well as higher dispersion is beneficial for improving catalytic activity of iron-based catalyst,4,8 and many efforts have been made by using Fe3O4 as the active phase in the previous work.26,41,42| Reaction | Rate constant at pH = 3 (mol−1 s−1) | |
|---|---|---|
| 1 | Fe2+ + H2O2 → Fe3+ + ˙OH + OH− | 63 |
| 2 | H2O2 + Fe3+ → Fe2+ + HO2˙/O2˙− + H+ | 2.0 × 10−3 |
| 3 | ˙OH + H2O2 → HO2˙/O2˙− + H2O | 3.3 × 107 |
| 4 | Fe3+ + HO2˙/O2˙− → Fe2+ + O2 + H+ | 7.8 × 105 |
| 5 | Fe2+ + ˙OH → Fe3+ + OH− | 3.2 × 108 |
| 6 | HO2˙/O2˙− + HO2˙/O2˙− → H2O2 + H2O | 2.3 × 106 |
| 7 | ˙OH + HO2˙/O2˙− → H2O + O2 | 7.1 × 109 |
| 8 | ˙OH + ˙OH → H2O2 | 5.2 × 109 |
FeZSM-5, obtained from hydrothermal synthesis, is with the ODAs in its channel system and with good iron dispersion. The decomposition process of TPA+ used as ODAs in this work is shown in Fig. 12. The decomposition of TPA+ involves two gradual template decomposition steps. At a low temperature, tripropylamine (Pr3N) and propene obtained from a Hofmann elimination of TPA+. In a second step, Pr3N was further degraded into lower amines and consequently into propene. Finally, the unsaturated products like propene undergo oligomerization, cyclization and aromatization.30 The oligomerization of the template derivatives finally lead to the coke-like species which have relatively strong interaction with ZSM-5 framework. When FeZSM-5 was calcined in air, the coke-like species would likely convert easily to gaseous products with the help of O2 and iron species are seldom influenced. In contrast, when calcined in N2 atmosphere, the temperature for those carbon species to break up is much higher. Higher temperature leads to the conversion of iron species from framework to non-framework. At the same time, the coke-like species can play a role in reducing the non-framework iron species.
The strategy involving the organic structure directing agents as a reductant to tune the iron species is also reliable in other zeolites.
4 Conclusions
A superior FeZSM-5 catalyst with excellent dispersion and valence tunable iron species was obtained from hydrothermal synthesis utilizing tetrapropylammonium bromide as the ODA in its channel system. Through simple thermal treatment in nonoxidative atmosphere, some framework iron converts to non-framework species. At the same time, the coke-like species obtained from oligomerization of ODAs play an important role in tuning the iron species to metastable state which is good for phenol oxidation. The catalytic activity of samples calcined in N2 is totally higher than that in air and the phenol conversion can improve from 44% to 90% within 60 min. The strategy involving using the organic directing agents as a reductant to tune the iron species and using the confinement effect of microporous structure to get higher dispersion is a reliable method to prepare well dispersed zeolite-encapsulated iron oxide for phenol oxidation.Acknowledgements
This work was supported by the State Key Program of National Natural Science Foundation of China (grant no. 21236008).Notes and references
- F. C. Moreira, S. Garcia-Segura, R. A. R. Boaventura, E. Brillas and V. J. P. Vilar, Degradation of the antibiotic trimethoprim by electrochemical advanced oxidation processes using a carbon-PTFE air-diffusion cathode and a boron-doped diamond or platinum anode, Appl. Catal., B, 2014, 160–161, 492–505 CrossRef CAS.
- A. D. Bokare and W. Choi, Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS PubMed.
- M. A. Oturan and J.-J. Aaron, Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef CAS.
- P. V. Nidheesh, Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review, RSC Adv., 2015, 5, 40552–40577 RSC.
- W. R. P. Barros, J. R. Steter, M. R. V. Lanza and A. C. Tavares, Catalytic activity of Fe3-xCuxO4 nanoparticles for the degradation of Amaranth food dye by heterogeneous electro-Fenton process, Appl. Catal., B, 2016, 180, 434–441 CrossRef CAS.
- M. B. Kasiri, H. Aleboyeh and A. Aleboyeh, Degradation of Acid Blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst, Appl. Catal., B, 2008, 84, 9–15 CrossRef CAS.
- Y. Lei, C. S. Chen, Y. J. Tu, Y. H. Huang and H. Zhang, Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions, Environ. Sci. Technol., 2015, 49, 6838–6845 CrossRef CAS PubMed.
- X. Hu, B. Liu, Y. Deng, H. Chen, S. Luo, C. Sun, P. Yang and S. Yang, Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution, Appl. Catal., B, 2011, 107, 274–283 CrossRef CAS.
- Y. Wang, H. Zhao and G. Zhao, Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants, Appl. Catal., B, 2015, 164, 396–406 CrossRef CAS.
- X. Li, X. Liu, L. Xu, Y. Wen, J. Ma and Z. Wu, Highly dispersed Pd/PdO/Fe2O3 nanoparticles in SBA-15 for Fenton-like processes: confinement and synergistic effects, Appl. Catal., B, 2015, 165, 79–86 CrossRef CAS.
- L. Luo, C. Dai, A. Zhang, J. Wang, M. Liu, C. Song and X. Guo, A facile strategy for enhancing FeCu bimetallic promotion for catalytic phenol oxidation, Catal. Sci. Technol., 2015, 5, 3159–3165 CAS.
- C. Dai, A. Zhang, L. Li, K. Hou, F. Ding, J. Li, D. Mu, C. Song, M. Liu and X. Guo, Synthesis of Hollow Nanocubes and Macroporous Monoliths of Silicalite-1 by Alkaline Treatment, Chem. Mater., 2013, 25, 4197–4205 CrossRef CAS.
- A. Cihanoğlu, G. Gündüz and M. Dükkancı, Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites, Appl. Catal., B, 2015, 165, 687–699 CrossRef.
- C. H. Christensen, I. Schmidt, A. Carlsson, K. Johannsen and K. herbst, Crystals in crystals-nanocrystals within mesoporous zeolite single crystals, J. Am. Chem. Soc., 2005, 127, 8098–8102 CrossRef CAS PubMed.
- L. Luo, C. Dai, A. Zhang, J. Wang, M. Liu, C. Song and X. Guo, Facile synthesis of zeolite-encapsulated iron oxide nanoparticles as superior catalysts for phenol oxidation, RSC Adv., 2015, 5, 29509–29512 RSC.
- Y. Wei, T. E. Parmentier, K. P. de Jong and J. Zecevic, Tailoring and visualizing the pore architecture of hierarchical zeolites, Chem. Soc. Rev., 2015, 44, 7234–7261 RSC.
- J. Shi, On the synergetic catalytic effect in heterogeneous nanocomposite catalysts, Chem. Rev., 2013, 113, 2139–2181 CrossRef CAS PubMed.
- C. Dai, A. Zhang, J. Li, K. Hou, M. Liu, C. Song and X. Guo, Synthesis of yolk-shell HPW@Hollow silicalite-1 for esterification reaction, Chem. Commun., 2014, 50, 4846–4848 RSC.
- C. Li, R. Younesi, Y. Cai, Y. Zhu, M. Ma and J. Zhu, Photocatalytic and antibacterial properties of Au-decorated Fe3O4@mTiO2 core–shell microspheres, Appl. Catal., B, 2014, 156–157, 314–322 CrossRef CAS.
- Y. Wang, H. Sun, X. Duan, H. M. Ang, M. O. Tadé and S. Wang, A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol, Appl. Catal., B, 2015, 172–173, 73–81 CrossRef CAS.
- Y. Gao, Y. Wang and H. Zhang, Removal of Rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation, Appl. Catal., B, 2015, 178, 29–36 CrossRef CAS.
- B. Dutta, S. Jana, A. Bhattacharjee, P. Gütlich, S. Iijima and S. Koner, γ-Fe2O3 nanoparticle in NaY-zeolite matrix: Preparation, characterization, and heterogeneous catalytic epoxidation of olefins, Inorg. Chim. Acta, 2010, 363, 696–704 CrossRef CAS.
- Z. Ai, Z. Gao, L. Zhang, W. He and J. Yin, Core–Shell Structure Dependent Reactivity of Fe@Fe2O3 Nanowires on Aerobic Degradation of 4-Chlorophenol, Environ. Sci. Technol., 2013, 47, 5344–5352 CrossRef CAS PubMed.
- M. Dükkancı, G. Gündüz, S. Yılmaz, Y. C. Yaman, R. V. Prikhod'ko and I. V. Stolyarova, Characterization and catalytic activity of CuFeZSM-5 catalysts for oxidative degradation of Rhodamine 6G in aqueous solutions, Appl. Catal., B, 2010, 95, 270–278 CrossRef.
- M. Xia, M. Long, Y. Yang, C. Chen, W. Cai and B. Zhou, A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution, Appl. Catal., B, 2011, 110, 118–125 CrossRef CAS.
- L. Xu and J. Wang, Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles, Appl. Catal., B, 2012, 123–124, 117–126 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schuth, Magnetic nanoparticles: synthesis, protection, functionalization, and application, Angew. Chem., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- J. Perez-Ramirez, M. S. Kumar and A. Bruckner, Reduction of N2O with CO over FeMFI zeolites: influence of the preparation method on the iron species and catalytic behavior, J. Catal., 2004, 223, 13–27 CrossRef CAS.
- A. Janda and A. T. Bell, Effects of Si/Al ratio on the distribution of framework Al and on the rates of alkane monomolecular cracking and dehydrogenation in H-MFI, J. Am. Chem. Soc., 2013, 135, 19193–19207 CrossRef CAS PubMed.
- L. Karwacki and B. M. Weckhuysen, New insight in the template decomposition process of large zeolite ZSM-5 crystals: an in situ UV-Vis/fluorescence micro-spectroscopy study, Phys. Chem. Chem. Phys., 2011, 13, 3681–3685 RSC.
- O. Prokopova, B. Bernauer, M. Frycova, P. Hrabanek, A. Zikanova and M. Kocirik, Principal Features of Tetrapropylammonium Hydroxide Removal Kinetics from Silicalite-1 in Quasi-isothermal Heating Regimes, J. Phys. Chem. C, 2013, 117, 1468–1476 CAS.
- L. E. Iton, R. B. Beal and D. T. Hodul, A new approach to the generation of metal-bearing, medium-pore, shape-selective zeolites for fischer-tropsch catalysis: spectroscopic studies of zeolites, J. Mol. Catal., 1983, 21, 151–171 CrossRef CAS.
- S. Bordiga, R. Buzzoni, F. Geobaldo, C. Lamberti, A. Zecchina, G. Leofanti, G. Petrini, G. Tozzola and G. Vlaic, Structure and Reactivity of Framework and Extraframework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods, J. Catal., 1996, 158, 485–501 CrossRef.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- Z. Xiao, S. Jin, X. Wang, W. Li, J. Wang and C. Liang, Preparation, structure and catalytic properties of magnetically separable Cu–Fe catalysts for glycerol hydrogenolysis, J. Mater. Chem., 2012, 22, 16598 RSC.
- G. F. Goya and H. R. Rechenberg, Superparamagnetic transition and local disorder in CuFe2O4 nanoparticles, Nanostruct. Mater., 1998, 10, 1001–1011 CrossRef CAS.
- G. Moretti, G. Fierro, G. Ferraris, G. B. Andreozzi and V. Naticchioni, N2O decomposition over [Fe]-MFI catalysts: Influence of the FexOy nuclearity and the presence of framework aluminum on the catalytic activity, J. Catal., 2014, 318, 1–13 CrossRef CAS.
- S. M. Maier, A. Jentys, E. Metwalli, P. Müller-Buschbaum and J. A. Lercher, Determination of the Redox Processes in FeBEA Catalysts in NH3-SCR Reaction by Mössbauer and X-ray Absorption Spectroscopy, J. Phys. Chem. Lett., 2011, 2, 950–955 CrossRef CAS.
- J. Taboada, A. Overweg, P. Kooyman, I. Arends and G. Mul, Following the evolution of iron from framework to extra-framework positions in isomorphously substituted [Fe,Al]MFI with Fe Mossbauer spectroscopy, J. Catal., 2005, 231, 56–66 CrossRef CAS.
- M. Hartmann, S. Kullmann and H. Keller, Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials, J. Mater. Chem., 2010, 20, 9002 RSC.
- T. Zhou, X. Wu, J. Mao, Y. Zhang and T.-T. Lim, Rapid degradation of sulfonamides in a novel heterogeneous sonophotochemical magnetite-catalyzed Fenton-like (US/UV/Fe3O4/oxalate) system, Appl. Catal., B, 2014, 160–161, 325–334 CrossRef CAS.
- H. Zhao, Y. Wang, Y. Wang, T. Cao and G. Zhao, Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism, Appl. Catal., B, 2012, 125, 120–127 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03552c |
| This journal is © The Royal Society of Chemistry 2016 |