A diiodo-BODIPY postmodified metal–organic framework for efficient heterogeneous organo-photocatalysis†
Ying Quana,
Qiu-Yan Li*a,
Quan Zhangb,
Wen-Qiang Zhanga,
Han Lua,
Jun-Hao Yua,
Jian Chena,
Xinsheng Zhao*c and
Xiao-Jun Wang*a
aJiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, School of Chemistry and Chemical Engineering, Jiangsu Normal University, Xuzhou 221116, P. R. China. E-mail: xjwang@jsnu.edu.cn; qyli@jsnu.edu.cn
bKey Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, P. R. China
cSchool of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou 221116, P. R. China. E-mail: xinshengzhao@jsnu.edu.cn
First published on 25th February 2016
Abstract
The organic photosensitizer diiodo-BODIPY has been covalently conjugated into a Zr(IV)-based metal–organic framework with UiO topology via postsynthetic modification, which serves as a highly active and recyclable heterogeneous photocatalyst for aerobic cross dehydrogenative coupling and oxidation/[3 + 2]cycloaddition reactions under visible light irradiation.
In the past few years, visible light mediated photoredox catalysis has received considerable attention, on account of the green and sustainable method for a variety of organic transformations under milder reaction conditions.1,2 Moreover, it can generate some sophisticated molecules that are not easily accessible by conventional thermal reactions. Typically, the photoredox catalysts include noble metal Ru, Ir and Pt complexes,1,3 as well as some organic dyes,4 such as eosin Y and Rose Bengal. As most of the reactions were performed homogenously, it is therefore difficult to recycle the catalysts for reuse. Clearly, a straightforward solution for this challenge is the immobilization of photo-catalysts onto insoluble solid-state materials, resulting in a heterogeneous catalyst. In this regard, several types of materials, such as silicas,5 polymers,6 and metal–organic frameworks (MOFs),7 have been employed to achieve this purpose. For example, Zhao's group has successfully incorporated [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) into organosilica for three photocatalytic transformations.5a Cooper and coworkers prepared Rose Bengal conjugated microporous polymers for aza-Henry reactions.6c Yu and Cohen reported a Zr-based MOF UiO-67 decorating with [Ru(bpy)3]2+ for aerobic oxidation of arylboronic acids under visible light irradiation.7d
Among these materials, MOFs as platforms for immobilizing catalysts are very attractive because of their highly tunable nature.8 Especially, the postsynthetic modification (PSM) strategy allows various functional groups to be readily tagged into MOFs.9 However, there are limited examples of MOFs with attached photosensitizers for photocatalytic organic transformations.7,10 Thus, it is urgent to develop an effective and versatile method for introducing photocatalysts into MOFs, with a purpose of achieving highly active heterogeneous photo-catalysis. Thanks to their modular nature, it is readily easy to prepare MOFs with tagged reactive groups, such as amine and azide. This could provide many possibilities for incorporation of a photosensitizer into MOFs through various reactions, particularly for an organic photosensitizer. However, there are few examples in this regard.
UiO series of MOFs comprised of Zr6-cluster secondary building units and dicarboxylate linkers represent a very rare kind of highly stable and porous MOF materials.11 In this work, we chose the amine-tagged UiO-68 (Zr-MOF UiO-68-NH2) because of its large cavities.11 Then, an already established organic photocatalyst diiodo-BODIPY12 was covalently conjugated into the above Zr-MOF (Fig. 1). Herein, we reported the example to validate the power of PSM for immobilizing photosensitizers within MOFs, which results in a highly active and recyclable heterogeneous photocatalyst for various organic transformations under visible light.
 | ||
| Fig. 1 Schematic representation of preparation for UiO-68-BP. Inset: the photographs of UiO-68-NH2 (left) and UiO-68-BP (right) under ambient light. | ||
The amine-tagged Zr-MOF UiO-68-NH2 can be prepared in a large scale by our modified literature method.13 A N,N′-dimethylformamide (DMF) solution of aminotriphenyldicarboxylic acid (amino-TPDC) and ZrCl4 in the presence of HAc and water was heated to 100 °C for 2 days, giving the desired MOFs (for detail see ESI†). As expected, powder X-ray diffraction (XRD) of as-synthesized products is similar to the simulated pattern from its single-crystal structure,14 confirming the phase purity and UiO structural framework. Scanning electron microscopy (SEM) image reveals their hexagonal plate-like morphologies with diameter at ∼100 nm and thickness at ∼30 nm (Fig. 2a). Photocatalyst diiodo-BODIPY was covalently incorporated into UiO-68-NH2 by the mild reaction between acyl chloride activated carboxylic group of organic species and amino group of bridging ligand, affording a deeply red solid UiO-68-BP (Fig. 1 and ESI for detail†). Moreover, the post-modified UiO-68-BP still retained the original hexagonal plate-like morphologies and powder XRD pattern of its parent MOF (Fig. 2b and d).
 | ||
| Fig. 2 Typical SEM images for UiO-68-NH2 (a), UiO-68-BP (fresh, (b)) and UiO-68-BP after catalysis (c); (d) powder XRD patterns for UiO-68-NH2 and UiO-68-BP. Scale bar: 100 nm. | ||
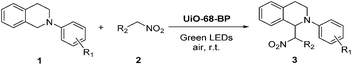
To verify that the immobilized diiodo-BODIPY in MOFs can still work as an effective photoredox catalyst, the aerobic cross dehydrogenative coupling (CDC) reaction of N-phenyl-1,2,3,4-tetrahydroisoquinoline (1a) with nitromethane (2a) in presence of air was performed as a model (Table 1). To our delight, the mixture of 1a and 2a containing catalytic amount UiO-68-BP under the irradiation of green LEDs (λmax = 520–530 nm) at room temperature for several hours, the formation of the desired cross-coupling product 3a was observed in a satisfied yield (Table 1, entry 1–4). No significant improvement could be detected with increase of catalyst amount (Table 1, entry 5). Other samples with lower loading amount of diiodo-BODIPY exhibited lower catalytic activities (Table 1, entry 6–7). Control experiments demonstrated that each component of the reaction is critical to the reaction efficiency (Table 1, entry 8–12).
| Entry | Conditions | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol) and 2a (1.0 mL), green LEDs (λ = 520–530 nm, 3 W). The reaction was conducted in an air atmosphere at room temperature.b Yields were determined by 1H NMR spectroscopy. n.r. = no reaction.c UiO-68-BP′ and UiO-68-BP′′ refer to ∼2% and ∼7% of organic linkers in MOF was functionalized by diiodo-BODIPY, respectively.d The reaction was performed in the presence of UiO-68-NH2.e The reaction mixture was stirred for 6 hours in the dark.f Degassed by nitrogen before irradiation. | |||
| 1 | UiO-68-BP (1 mg) | 2 | 72 |
| 2 | UiO-68-BP (1 mg) | 3 | 79 |
| 3 | UiO-68-BP (2 mg) | 2 | 76 |
| 4 | UiO-68-BP (2 mg) | 3 | 88 |
| 5 | UiO-68-BP (5 mg) | 3 | 89 |
| 6c | UiO-68-BP′ (2 mg) | 3 | 61 |
| 7c | UiO-68-BP′′ (2 mg) | 3 | 77 |
| 8d | UiO-68-NH2 (5 mg) | 3 | Trace |
| 9 | No catalyst | 3 | Trace |
| 10e | No light | — | n.r. |
| 11f | No O2 | 3 | n.r. |
With this initial success, we subsequently screened the reaction scope (Tables 2 and S1 in ESI†). Various substituted 1,2,3,4-tetrahydroisoquinoline derivatives can undergo the CDC reaction with nitromethane efficiently, giving the products in good to excellent yields (Table 2, 3a–3g). Moreover, the utilization of other nucleophiles, including nitroethane, nitropropane and dialkyl malonates, was also able to yield the desired products smoothly (Table 2, 3h–3l and Table S1 in ESI†).
| a Reaction conditions: 1 (0.1 mmol), 2 (1 mL) and UiO-68-BP (2 mg) under air at room temperature, and the irradiation time was 3 h. Yields were determined by 1H NMR. Ratios of the two diastereoisomers are given in parentheses. |
|---|
 |
Meanwhile, the recyclability of UiO-68-BP as a heterogeneous catalyst was also investigated, which can be readily recovered from the reaction system by simple centrifugation. It was reused three photocatalysis recycles without obvious loss of catalytic activity (88%, 78% and 72%, respectively). The little decrease of yield should be attributed some minor decompositions of the organic photosensitizer diiodo-BODIPY caused by the long irradiation. However, SEM and powder XRD investigations of the recycled photocatalyst revealed that it still maintain the crystalline structure and framework as well as morphologies (Fig. 2c and d), mainly due to the highly stable UiO frameworks.
As previously reported, BODIPYs attached heavy atoms serves as a good photosensitizer for producing singlet oxygen (1O2), because of the enhanced intersystem crossing capacity.15 However, it was recently proposed that the superoxide radical anion (O2−˙) act as the active species in photo-promoted aerobic CDC reactions.2e,2f,4e Thus, electron spin resonance (ESR) measurements were performed to make it clear, in which 2,2,6,6-tetramethyl-1-piperidine (TEMP) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) were employed to capture 1O2 and O2−˙, respectively. As shown in Fig. 3, an expected characteristic signal of 1O2 was observed upon irradiation of the CH3CN mixture of UiO-68-BP and TEMP in air by green LEDs. However, the 1O2 signal could not be detected when 1a was added into the mixture. In contrast, there was no signal when DMPO was added into the CH3CN mixture of UiO-68-BP, however, a characteristic signal of O2−˙ trapped by DMPO was clearly observed upon addition of 1a. These above results indicate that although diiodo-BODIPY is a reported efficient sensitizer in the production of 1O2,15 however, this process is highly suppressed by the effective single electron transfer (SET) between 1a and excited diiodo-BODIPY (PS*) in the present case, along with the generation of the radical cation 1a˙+ and the radical anion PS−˙. Subsequently, the generated radical anion PS−˙ reacts with molecular oxygen to produce the superoxide radical anion O2−˙, which is a crucial intermediate in the reaction. Based on the above analysis, a similar and plausible mechanism was proposed in Scheme S3 (in ESI†).2e,2f,4e Thus, these results confirmed that there still was the efficient SET process between the immobilized photocatalyst and substrate molecules.
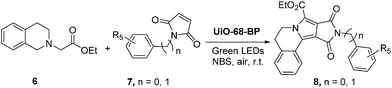
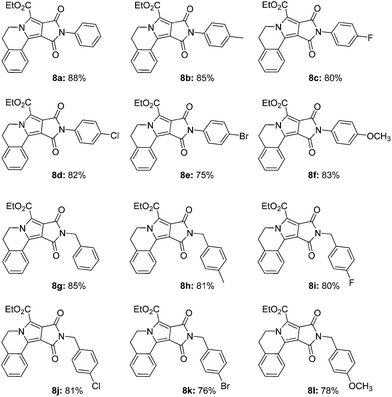
In order to further explore the applications of such immobilized photocatalyst in MOFs, a series of oxidation–[3 + 2]cycloaddition–aromatization tandem reaction of 6 with 7 catalyzed by UiO-68-BP was performed. As shown in Table 3, most of the final pyrrolo[2,1-a]isoquinoline products were obtained in satisfactory yields. Moreover, other organic transformations mediated by oxygen, including aerobic amine coupling and aerobic oxidation of arylboronic acid and thioanisole, were also successfully and efficiently conducted in the presence of UiO-68-BP, indicating its a wide range of applications in organic photocatalysis (in ESI†).
| a Reaction conditions: a 1 mL CH3CN solution of 6 (0.12 mmol), 7 (0.10 mmol) and UiO-68-BP (2 mg) was irradiated for 2 hours under air at room temperature; then, NBS (0.12 mmol) was added into the reaction mixture, which was stirred for further 10 min. Yields were determined by 1H NMR. |
|---|
 |
Conclusions
In summary, we have reported an organic photosensitizer diiodo-BODIPY postmodified metal–organic framework, which was used as a highly active and recyclable heterogeneous photocatalyst for aerobic cross dehydrogenative coupling and oxidation–[3 + 2]cycloaddition–aromatization tandem reactions as well as other oxidative reactions under visible light irradiation. The example demonstrated in this work has validated that the postsynthetic modification (PSM) strategy of MOFs can serve as a versatile and powerful method for incorporation of photosensitizers into MOFs. Moreover, it is expected that more heterogeneous photocatalysts based on MOFs with excellent photoredox properties will be constructed for promoting a wider scope of organic transformations by using visible light.Acknowledgements
The work is financially supported from National Natural Science Foundation of China (21302072, 51403081 and 21376113), Natural Science Foundation of Jiangsu Province (BK20130226 and BK20140137), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Major Projects of Natural Science Research in Jiangsu Province (15KJA150004).Notes and references
- (a) J. W. Beatty and C. R. J. Stephenson, Acc. Chem. Res., 2015, 48, 1474–1484 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (d) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS PubMed; (e) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687–7697 RSC; (f) E. Meggers, Chem. Commun., 2015, 51, 3290–3301 RSC.
- (a) J. D. Cuthbertson and D. W. C. MacMillan, Nature, 2015, 519, 74–77 CrossRef CAS PubMed; (b) J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff and D. W. C. MacMillan, Nature, 2015, 524, 330–334 CrossRef CAS PubMed; (c) G. Zhang, C. Liu, H. Yi, Q. Meng, C. Bian, H. Chen, J.-X. Jian, L.-Z. Wu and A. Lei, J. Am. Chem. Soc., 2015, 137, 9273–9280 CrossRef CAS PubMed; (d) Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052–19055 CrossRef CAS PubMed; (e) X.-Z. Wang, Q.-Y. Meng, J.-J. Zhong, X.-W. Gao, T. Lei, L.-M. Zhao, Z.-J. Li, B. Chen, C.-H. Tung and L.-Z. Wu, Chem. Commun., 2015, 51, 11256–11259 RSC; (f) J.-J. Zhong, Q.-Y. Meng, G.-X. Wang, Q. Liu, B. Chen, K. Feng, C.-H. Tung and L.-Z. Wu, Chem.–Eur. J., 2013, 19, 6443–6450 CrossRef CAS PubMed; (g) W. Guo, L.-Q. Lu, Y. Wang, Y.-N. Wang, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 2265–2269 CrossRef CAS PubMed; (h) J. Xuan, T.-T. Zeng, Z.-J. Feng, Q.-H. Deng, J.-R. Chen, L.-Q. Lu, W.-J. Xiao and H. Alper, Angew. Chem., Int. Ed., 2015, 54, 1625–1628 CrossRef CAS PubMed; (i) M. Nakajima, E. Fava, S. Loescher, Z. Jiang and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 8828–8832 CrossRef CAS PubMed; (j) H. Huo, C. Wang, K. Harms and E. Meggers, J. Am. Chem. Soc., 2015, 137, 9551–9554 CrossRef CAS PubMed; (k) X.-B. Li, Z.-J. Li, Y.-J. Gao, Q.-Y. Meng, S. Yu, R. G. Weiss, C.-H. Tung and L.-Z. Wu, Angew. Chem., Int. Ed., 2014, 53, 2085–2089 CrossRef CAS PubMed.
- (a) H. Huo, X. Shen, C. Wang, L. Zhang, P. Roese, L.-A. Chen, K. Harms, M. Marsch, G. Hilt and E. Meggers, Nature, 2014, 515, 100–103 CrossRef CAS PubMed; (b) S. Z. Tasker and T. F. Jamison, J. Am. Chem. Soc., 2015, 137, 9531–9534 CrossRef CAS PubMed; (c) Q.-Y. Meng, T. Lei, L.-M. Zhao, C.-J. Wu, J.-J. Zhong, X.-W. Gao, C.-H. Tung and L.-Z. Wu, Org. Lett., 2014, 16, 5968–5971 CrossRef CAS PubMed.
- (a) D. Ravelli and M. Fagnoni, ChemCatChem, 2012, 4, 169–171 CrossRef CAS; (b) D. P. Hari and B. Koenig, Chem. Commun., 2014, 50, 6688–6699 RSC; (c) S. Fukuzumi and K. Ohkubo, Org. Biomol. Chem., 2014, 12, 6059–6071 RSC; (d) T.-T. Zeng, J. Xuan, W. Ding, K. Wang, L.-Q. Lu and W.-J. Xiao, Org. Lett., 2015, 17, 4070–4073 CrossRef CAS PubMed; (e) Q. Liu, Y.-N. Li, H.-H. Zhang, B. Chen, C.-H. Tung and L.-Z. Wu, Chem.–Eur. J., 2012, 18, 620–627 CrossRef CAS PubMed.
- (a) A. Jana, J. Mondal, P. Borah, S. Mondal, A. Bhaumik and Y. Zhao, Chem. Commun., 2015, 51, 10746–10749 RSC; (b) S. Guo, H. Zhang, L. Huang, Z. Guo, G. Xiong and J. Zhao, Chem. Commun., 2013, 49, 8689–8691 RSC; (c) G. J. Barbante, T. D. Ashton, E. H. Doeven, F. M. Pfeffer, D. J. D. Wilson, L. C. Henderson and P. S. Francis, ChemCatChem, 2015, 7, 1655–1658 CrossRef CAS.
- (a) Q. Sun, Z. F. Dai, X. J. Meng and F. S. Xiao, Chem. Soc. Rev., 2015, 44, 6018–6034 RSC; (b) Z. Xie, C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 2056–2059 CrossRef CAS PubMed; (c) J.-X. Jiang, Y. Li, X. Wu, J. Xiao, D. J. Adams and A. I. Cooper, Macromolecules, 2013, 46, 8779–8783 CrossRef CAS.
- (a) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC; (b) S. Wang and X. Wang, Small, 2015, 11, 3097–3112 CrossRef CAS PubMed; (c) C. Wang, Z. Xie, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445–13454 CrossRef CAS PubMed; (d) X. Yu and S. M. Cohen, Chem. Commun., 2015, 51, 9880–9883 RSC.
- (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed; (b) M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed; (c) H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- (a) S. M. Cohen, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS PubMed; (b) Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC; (c) S. M. Cohen, Chem. Sci., 2010, 1, 32–36 RSC; (d) A. D. Burrows and S. M. Cohen, CrystEngComm, 2012, 14, 4095–4095 RSC.
- (a) M.-H. Xie, X.-L. Yang, C. Zou and C.-D. Wu, Inorg. Chem., 2011, 50, 5318–5320 CrossRef CAS PubMed; (b) P. Wu, C. He, J. Wang, X. Peng, X. Li, Y. An and C. Duan, J. Am. Chem. Soc., 2012, 134, 14991–14999 CrossRef CAS PubMed; (c) J. A. Johnson, J. Luo, X. Zhang, Y.-S. Chen, M. D. Morton, E. Echeverría, F. E. Torres and J. Zhang, ACS Catal., 2015, 5, 5283–5291 CrossRef CAS; (d) W.-Q. Zhang, Q.-Y. Li, Q. Zhang, Y. Lu, H. Lu, W. Wang, X. Zhao and X.-J. Wang, Inorg. Chem., 2016, 55, 1005–1007 CrossRef CAS PubMed.
- (a) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed; (b) M. Kim and S. M. Cohen, CrystEngComm, 2012, 14, 4096–4104 RSC.
- (a) L. Huang and J. Zhao, RSC Adv., 2013, 3, 23377–23388 RSC; (b) S. Guo, R. Tao and J. Zhao, RSC Adv., 2014, 4, 36131–36139 RSC; (c) J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- (a) C. He, K. Lu, D. Liu and W. Lin, J. Am. Chem. Soc., 2014, 136, 5181–5184 CrossRef CAS PubMed; (b) C. He, K. Lu and W. Lin, J. Am. Chem. Soc., 2014, 136, 12253–12256 CrossRef CAS PubMed.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- (a) T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed; (b) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC; (c) S. G. Awuah and Y. You, RSC Adv., 2012, 2, 11169–11183 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, additional synthesis and characterization data. See DOI: 10.1039/c6ra03516g |
| This journal is © The Royal Society of Chemistry 2016 |