Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay†
A. Rajeshwari,
S. Suresh,
Natarajan Chandrasekaran and
Amitava Mukherjee*
Centre for Nanobiotechnology, VIT University, Vellore 632014, India. E-mail: amit.mookerjea@gmail.com; amitav@vit.ac.in; Tel: +91 416 220 2620
First published on 26th February 2016
Abstract
The progressive increase in the usage of gold nanoparticles (NPs) in industrial and commercial products leads to the potential release of nanoparticles into the environment, which could cause adverse effects on living systems. In the present work, the size- and dose-dependent cytogenetic effects of gold NPs towards a plant system were evaluated for the first time by a simple and cost-effective Allium cepa (A. cepa) bioassay. Citrate-capped gold NPs of three different sizes, 15 (Au15), 30 (Au30), and 40 (Au40) nm, were synthesized by a citrate reduction method. The mean hydrodynamic diameter and morphology of as-synthesized gold NPs were characterized by dynamic light scattering and transmission electron microscopy analyses. Several chromosomal aberrations were observed under an optical microscope upon the exposure of A. cepa root tip cells with 0.1, 1, and 10 μg mL−1 of Au15, Au30, and Au40. The mitotic indices in treated root tips were directly proportional to the NP concentration and inversely related to their size. The vehicle control (citrate) had no impact on the mitotic index. Furthermore, the effects of gold NPs on the A. cepa root tip were confirmed by analyzing the generation of various reactive oxidant species (hydroxyl, superoxide and hydrogen peroxide), which possibly led to lipid peroxidation in the system.
1. Introduction
The production or manufacture of nanoscale materials has increased substantially in recent years. Engineered nanoparticles find a wide range of applications in different areas like materials engineering, energy production, biosensing, biomedical and environmental remediation.1–5 The increase in the production and usage of the engineered nanoparticles for industrial applications involving commercial products eventually leads to their release into the environment. This will enhance the probability of the exposure of plants to the released nanoparticles.6 Due to their unique properties, these nanoscale particles have the ability to cross the cell barrier and interact with the intracellular structures leading to cellular and genetic toxicity. However, a knowledge gap in understanding the exact mechanism involved in the uptake, transport, and toxicity of nanoparticles in the plant systems still persists.7 Among the released nanoparticles, gold nanoparticles (NPs) are the most commonly synthesized metallic nanoparticles, and since the 16th century, they have been used for medical purposes. Owing to their ease of synthesis, functionalization, unique optical property, and chemical stability, gold NPs find their application in bioimaging,8 drug delivery,9 cancer treatment,10 and chemical sensing.11 Recently, gold NPs have been used as an efficient delivery system in plants to improve the agricultural yield.12 Therefore, it is crucial to understand the interactions of gold NPs with the plant system.Several reports have been found on the toxic effect of gold NPs in algae,13 bacteria,14 cell lines,15 mice,16 and aquatic organisms.17 The transport of gold NPs in plant cells is influenced by the nanoparticle size, shape, surface charge, and surface coating.18–20 The risk of gold NPs to humans has risen through their transfer and biomagnification in the food chain.21 The nanoparticles present in the environment can make contact with plants through water, air and/or soil.22,23 Sabo-Attwood et al., have reported the size-dependent uptake of gold NPs in tobacco. The uptake, distribution, and toxicity of gold NPs were found to be more for 3.5 nm AuNPs when compared to 18 nm AuNPs.24 Zhu et al. have studied the effect of surface charge on the uptake and distribution of gold NPs in plant systems (rice, radish, ryegrass, and pumpkin). This study has suggested that the positively charged gold NPs can readily be taken up by plant cells, whereas the negatively charged gold NPs could translocate to the plant shoot from the roots cells.20 The internalization of gold NPs in tomato plants has the potential to induce cellular toxic effects by changing the gene expression level.25 In contrast to the previous studies, gold NPs were found to act as a growth regulator and could enhance the total seed yield in Arabidopsis thaliana and Brassica juncea.26,27
The Allium cepa (A. cepa) test has been used a bioindicator of environmental pollution since 1920. A. cepa has been considered as an excellent in vivo model for studying the toxicity of nanoparticles as their roots are able to grow in direct contact with the effluent or any substance of interest, and they also possess a stable chromosome number and karyotype and exhibit diversity of chromosomal morphology, clear mitotic phases, rapid response to genotoxic materials, and the rare occurrences of spontaneous chromosomal damages.28 The A. cepa bioassay has been validated as an efficient and standard method for the in situ monitoring of environmental substances and chemical screening by United Nations Environment Programme (UNEP) and the International Programme on Chemical Safety (IPCS).29,30
For the last few years, A. cepa has been used as a bioindicator to evaluate the cytotoxic and genotoxic effects of metal oxide and metallic nanoparticles. Kumari et al. have reported that both silver nanoparticles (AgNPs) and zinc oxide nanoparticles (ZnO NPs) were found to induce chromosomal aberrations in A. cepa root tip cells at exposure concentrations of 25, 50, 75, and 100 μg mL−1 by causing oxidative stress.31,32 Panda et al. and Liman have also used the A. cepa bioassay to evaluate the genotoxic effect of AgNPs and bismuth(III) oxide nanoparticles.33,34 Ghosh et al. studied the role of lipid peroxidation caused by titania nanoparticles (TiO2 NPs) that can in turn result in DNA damage in A. cepa cells.35 Further, the study carried out by Pakrashi et al. has suggested that the uptake of TiO2 NPs in particulate form may induce the generation of reactive oxygen species and cause chromosomal aberration and DNA damage in A. cepa.36 The internalization of aluminum oxide (Al2O3) and chromium(III) oxide (Cr2O3) nanoparticles also has a cytogenetic effect on A. cepa meristematic cells at exposure concentrations of 0.01, 0.1, 1, 10, and 100 μg mL−1.37,38
A detailed literature survey reveals that the thorough toxicological analysis of gold NPs has not been validated up to date using the A. cepa bioassay. This is also the first-ever study to investigate the size- and dose-dependent impact of citrate-capped gold NPs on A. cepa root tip cells. There is a pressing need to develop simple and cost-effective bio assays for testing nanomaterial toxicity as a part of environmental risk assessment. Given the spiraling increase in the usage of gold NPs and the consequent elevated risk of environmental exposure, we were curious to find out whether A. cepa bioassay may be utilized to evaluate the toxicity of these particles. Various chromosomal aberrations were observed in the A. cepa root tip cells upon their exposure to different sizes of gold NPs (15, 30, and 40 nm), each size tested at 3 different concentrations of 0.1, 1, and 10 μg mL−1. The decrease in the percentage of mitotic index and chromosomal aberration was observed to be directly proportional to the concentration of nanoparticles and inversely proportional to their size. The toxic effects of gold NPs were further corroborated with the generation of various major oxidant species [hydroxyl, superoxide and hydrogen peroxide] in the system, which resulted in lipid peroxidation and consequent cellular damages.
2. Materials and methods
2.1. Chemicals
Hydrogen tetrachloroaurate hydrate (HAuCl4) and potassium iodide (KI) were obtained from SRL Pvt. Ltd (India). Hydrochloric acid (HCl), dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4), tris(hydroxymethyl) aminomethane hydrochloric acid (Tris–HCl), Nitro Blue Tetrazolium (NBT), nicotinamide adenine dinucleotide hydrogen (NADH), sucrose, trichloroacetic acid (TCA), 2-deoxy-D-ribose, thiobarbituric acid (TBA), and glacial acetic acid were obtained from Hi-Media Pvt. Ltd. (India). Acetocarmine was purchased from Nice chemicals Pvt. Ltd (India) and trisodium citrate was from SD Fine Chemicals Ltd. (India).2.2. Synthesis of gold NPs
2.3. Characterization of gold NPs
The synthesized gold NPs were subjected to UV-visible spectroscopic studies. The absorption spectrum of gold NPs was recorded by UV-visible spectrophotometer (Shimadzu UV- 2600, Japan) and the recorded spectra were then re-plotted using Microsoft Excel. The particle size along with the polydispersity of as-synthesized gold NPs without further modification was determined using a particle size analyzer (90 Plus Particle Analyzer, Brookhaven Instruments Corporation, NY, USA). The particle size of the nanoparticles was derived by calculating the mean hydrodynamic diameter (MHD) from the autocorrelation function of the intensity of light scattered from the particles undergoing Brownian motion. Further, the morphology of as-synthesized gold NPs was characterized by transmission electron microscopy (FEI Tecnai T20 S-TWIN TEM). The concentration of citrate-capped gold NPs was analyzed by inductively-coupled plasma-optical emission spectroscopy (ICP-OES; Perkin Elmer Optima 5300 DV, USA).2.4. Test system and treatment
The toxicity study was performed by the as-synthesized gold NPs without further modification. Healthy onion bulbs, weighing 30–35 g each, were grown under dark conditions in an enclosed chamber. A temperature of 28 ± 2 °C was maintained, and renewed water supply was provided for every 24 h. Roots of 2-to-3 cm length incise from the bulb were treated with various concentrations (0.1, 1, and 10 μg mL−1) of the three different gold NP colloidal solution and capping agent (136 mM) for 4 h. Five replicates were made for each concentration for statistical validation of the observations.362.5. Microscopic analysis
The root tips were removed and rinsed with distilled deionized water after 4 h interaction of root tips with various concentrations (0.1, 1, and 10 μg mL−1) of Au15, Au30, and Au40. Then, the root tips were immersed in 1 N HCl for 20 min and dipped in acetocarmine stain for 5 min. After the 5 min incubation, the roots were removed and 1–2 mm was cut from the tip. The stained root tips were placed onto a glass slide, covered with a coverslip, and was pressed (squashed) firmly with the help of a thumb to prepare a uniform squash. The slides were observed under an optical microscope (Axiostar, Zeiss, Germany) for cytological changes at 1000× magnification.36The toxic effect of gold NPs on A. cepa root tip cells was determined by scoring 1000 cells per test concentration. The mitotic index and phase index were calculated as follows.42
TDC – total dividing cells, TC – total count.
2.6. Reactive oxygen species (ROS) analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min at 4 °C. The supernatant was collected in a fresh tube without disturbing the pellet, and 0.5 mL of the supernatant was mixed with 0.5 mL of potassium phosphate buffer (10 mM) and 1 mL of potassium iodide. The H2O2 content in the supernatant was measured by comparing its absorbance at 390 nm with a standard calibration curve drawn using solutions with known H2O2 concentrations and is expressed in μmoles g−1 of fresh weight (FW).44
000 rpm for 20 min at 4 °C. The supernatant was collected in a fresh tube without disturbing the pellet, and 0.5 mL of the supernatant was mixed with 0.5 mL of potassium phosphate buffer (10 mM) and 1 mL of potassium iodide. The H2O2 content in the supernatant was measured by comparing its absorbance at 390 nm with a standard calibration curve drawn using solutions with known H2O2 concentrations and is expressed in μmoles g−1 of fresh weight (FW).44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min, and the supernatants were collected and incubated at 37 °C for 2 h. To 500 μL of the supernatant, a reaction mixture containing 3 mL of 0.5% (w/v) thiobarbituric acid and 1 mL glacial acetic acid (v/v) was added, and the solution was subjected to heating to 100 °C for 30 min and cooled down to 4 °C for 10 min. The concentration of malondialdehyde (MDA) was calculated using its extinction coefficient (155 mM−1 cm−1) by measuring the absorbance at 532 nm and is expressed in μmoles g−1 of fresh weight.45
000 rpm for 15 min, and the supernatants were collected and incubated at 37 °C for 2 h. To 500 μL of the supernatant, a reaction mixture containing 3 mL of 0.5% (w/v) thiobarbituric acid and 1 mL glacial acetic acid (v/v) was added, and the solution was subjected to heating to 100 °C for 30 min and cooled down to 4 °C for 10 min. The concentration of malondialdehyde (MDA) was calculated using its extinction coefficient (155 mM−1 cm−1) by measuring the absorbance at 532 nm and is expressed in μmoles g−1 of fresh weight.452.7. Lipid peroxidation
1.5 g of fresh roots were homogenized with 3 mL reaction mixture containing 20% (w/v) trichloroacetic acid and 0.5% (w/v) thiobarbituric acid. The homogenate was incubated at 95 °C for 30 min. After the 30 min incubation, the homogenate was placed in ice to stop the reaction and was subjected to centrifugation for 10 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was collected, and the absorbance readings were recorded at 532 and 600 nm. The non-specific absorbance at 600 nm was subtracted from 532 nm and the concentration of malondialdehyde was determined using its extinction coefficient (155 mM−1 cm−1). The amount of malondialdehyde was directly proportional to the lipid peroxidation generation level.46
000 rpm. The supernatant was collected, and the absorbance readings were recorded at 532 and 600 nm. The non-specific absorbance at 600 nm was subtracted from 532 nm and the concentration of malondialdehyde was determined using its extinction coefficient (155 mM−1 cm−1). The amount of malondialdehyde was directly proportional to the lipid peroxidation generation level.46
2.8. Statistical analysis
Experiments were carried out in triplicates, and the mean value of the results with standard error is reported, the significant difference between the three sizes of gold NPs for each test concentrations was confirmed with respect to the control samples by two-way analysis of variance (ANOVA) using GraphPad Prism 5.0 software.3. Results and discussion
3.1. Characterization of gold NPs
The three sizes of gold NPs, Au15, Au30, and Au40, were synthesized by direct and reverse Turkevich methods. The UV-visible spectra of synthesized gold NPs, Au15, Au30, and Au40, have shown distinct surface plasmon resonance peaks at 528, 526, and 527 nm (Fig. S1, ESI†), which confirmed the formation of gold NPs.47The sizes of as-synthesized gold NPs in the medium were analyzed by a particle size analyzer. The mean hydrodynamic diameters were found to be 15 ± 1 nm for Au15, 30 ± 1 nm for Au30, and 40 ± 1 nm for Au40 (Fig. 1a–c). The polydispersities were noted to be 0.236, 0.131, and 0.245 for Au15, Au30, and Au40, which confirms that the nanoparticles retained their monodisperse nature. The zeta potential measurements of Au15, Au30, and Au40 were found to be −34.33, −27.35, and −24.71 mV, and these results confirm that the gold NPs possessed negative surface charges.
 | ||
| Fig. 1 The particle size distribution (a–c) and TEM image (d–f) of as-synthesized gold NPs, Au15, Au30, and Au40 [MHD – mean hydrodynamic diameter]. | ||
Further, the size and morphology of nanoparticles were analyzed by transmission electron microscopy (TEM). Fig. 1d–f shows the TEM image of Au15, Au30, and Au40, which were used for our study. The TEM image reveals that the nanoparticles are monodispersed and spherically shaped, and the average particle sizes were 16.6, 31.7, and 39.7 nm for Au15, Au30, and Au40. The concentration of as-synthesized gold NPs determined by ICP-OES analysis were 25 μg mL−1 for Au15 and 10 μg mL−1 for Au30 and Au40 (Table S1, ESI†). The test concentrations of NPs were prepared by diluting the colloidal gold NPs in Milli-Q water.
3.2. Microscopic analysis
The toxic effects of citrate-capped gold NPs, Au15, Au30 and Au40, were determined based on their impact on the chromosome behavior and cell division in the meristematic cells of the root tip of A. cepa at different concentrations (0.1, 1, and 10 μg mL−1). Each treatment group including the treated and untreated had 5 replicates, and 1000 cells were scored for each replicate. In the control A. cepa root tip cells, different mitotic phases like prophase, metaphase, anaphase, and telophase were observed. The mitotic and phase indices calculated for the A. cepa root tip cells, before and after interaction with various concentrations (0.1, 1, and 10 μg mL−1), of Au15, Au30, and Au40, are shown in Table 1.| Treatment | P% | M% | A% | T% | MI% | CA% | |
|---|---|---|---|---|---|---|---|
| a Cells scored per test concentration (n = 5) – 1000 numbers, *(asterisk) represents the significance difference between control and NPs treated samples, ***P < 0.001, **P < 0.01, *P < 0.05, the symbol ‘#’ represents the significance difference between citrate and NPs treated samples, MI – mitotic index; SE – standard error; CA – chromosomal aberration; P, M, A, T stands for prophase, metaphase, anaphase and telophase as phase index in percentage, respectively. | |||||||
| Control (DI water) | 96.39 ± 0.51 | 1.36 ± 0.21 | 1.12 ± 0.25 | 1.12 ± 0.33 | 43.856 ± 1.07 | 0.09 ± 0.03 | |
| H2O2 | 95.53 ± 1.01 | 0.37 ± 0.13 | 4.03 ± 1.07 | 0.11 ± 0.11 | 17.92 ± 0.71 | 29.13 ± 4.71 | |
| Citrate | 38.8 mM | 98.69 ± 0.12 | 0.40 ± 0.10 | 0.56 ± 0.05 | 0.35 ± 0.05 | 40.63 ± 0.64 | 0.01 ± 0.00 |
| 136 mM | 99.16 ± 0.05 | 0.30 ± 0.12 | 0.40 ± 0.11 | 0.14 ± 0.05 | 40.63 ± 0.25 | 0.52 ± 0.25 | |
| 34.5 mM | 98.72 ± 0.15 | 0.36 ± 0.06 | 0.56 ± 0.06 | 0.36 ± 0.06 | 41.51 ± 0.55 | 0.01 ± 0.00 | |
| Au15 | 0.1 μg mL−1 | 94.45 ± 0.70 | 2.25 ± 0.38 | 1.45 ± 0.21 | 1.86 ± 0.74 | 30.06 ± 0.84***,# | 0.87 ± 0.30**,# |
| 1 μg mL−1 | 95.12 ± 0.62 | 2.17 ± 0.23 | 1.88 ± 0.41 | 0.84 ± 0.08 | 28.54 ± 2.61***,# | 1.03 ± 0.46***,# | |
| 10 μg mL−1 | 90.43 ± 2.06 | 3.23 ± 0.35 | 1.60 ± 0.21 | 4.74 ± 1.97 | 22.64 ± 1.86***,# | 1.96 ± 0.87***,# | |
| Au30 | 0.1 μg mL−1 | 87.13 ± 1.53 | 2.79 ± 0.98 | 2.43 ± 0.49 | 7.60 ± 2.32 | 32.80 ± 0.66***,# | 0.50 ± 0.00*** |
| 1 μg mL−1 | 88.77 ± 1.81 | 3.06 ± 0.61 | 1.66 ± 0.29 | 6.52 ± 1.24 | 28.95 ± 0.69***,# | 0.94 ± 0.00**,# | |
| 10 μg mL−1 | 86.44 ± 1.35 | 3.08 ± 0.33 | 0.69 ± 0.91 | 9.79 ± 1.16 | 27.00 ± 2.33***,# | 1.70 ± 0.30***,# | |
| Au40 | 0.1 μg mL−1 | 95.05 ± 1.77 | 2.65 ± 1.06 | 1.28 ± 0.60 | 1.02 ± 0.23 | 33.88 ± 1.04***,# | 0.74 ± 0.06***,# |
| 1 μg mL−1 | 96.60 ± 0.88 | 1.74 ± 0.50 | 0.58 ± 0.11 | 1.09 ± 0.51 | 32.73 ± 1.73***,# | 0.83 ± 0.11***,# | |
| 10 μg mL−1 | 90.43 ± 2.06 | 3.23 ± 0.35 | 1.06 ± 0.21 | 4.74 ± 1.97 | 29.64 ± 1.29***,# | 1.46 ± 0.60***,# | |
The mitotic index (MI) for control root tip cells was found to be 43.80 ± 1.07%. The calculated MI values of A. cepa root tip cells were found to 30.06 ± 0.83, 28.54 ± 2.61, and 22.64 ± 1.85% after interaction with 0.1, 1, and 10 μg mL−1 of Au15. Upon the exposure to 0.1, 1, and 10 μg mL−1 of Au30, the mitotic indices were found to be 32.80 ± 0.66, 28.90 ± 0.69, and 27.00 ± 2.33%. Similarly, the MI values of A. cepa root tip cells noted after the treatment with 0.1, 1, and 10 μg mL−1 of Au40 were 33.80 ± 1.03, 32.70 ± 1.72, and 29.60 ± 1.29%. A concentration-dependent decrease in the MI percentage was observed in the treated groups after exposure to 0.1, 1, and 10 μg mL−1 of Au15, Au30, and Au40 in comparison to the control cells. Notably, at all the exposure concentrations, the decrease in MI values caused by Au15, Au30, and Au40 was significantly different from that in the control root tip cells (P < 0.001). Upon increasing the size of gold NPs, the impact of NPs on the MI value was observed to decrease in the order of Au15 > Au30 > Au40. A statistically significant difference (P < 0.001) in the MI values was observed within the different sizes of gold NPs (Au15, Au30, and Au40) at all concentrations. A dose-dependent and size-dependent increase in the chromosomal aberration (CA) frequency was noted (Table 1).
The effect of citrate on mitotic index and chromosomal aberration was studied for 34.5, 38.8 and 136 mM concentration of trisodium citrate employed in the synthesis of gold NPs (Table 1). Upon the exposure to 38.8, 136 and 34.5 mM concentration of trisodium citrate, the mitotic index was found to be 40.63 ± 0.64, 40.63 ± 0.25 and 41.51 ± 0.55%. No significant difference was observed in MI% within the citrate concentrations. The trisodium citrate was found to cause the chromosomal aberrations by itself at higher concentration (136 mM). The MI percentage was found decrease and CA frequency was noted to increase significantly (P < 0.001) for 0.1, 1, and 10 μg mL−1 of Au15 treated root cells in comparison to the citrate concentration 38.8 mM used for the synthesis of Au15. Similar effect was also observed for the 0.1, 1, and 10 μg mL−1 of Au40 with respect to 34.5 mM citrate concentration. The increase in CA frequency in the gold NPs compared to citrate control (136 mM) was found to be significant at all concentration excluding 0.1 μg mL−1 of Au30 treated cells. Sabo-Attwood et al. have reported that the citrate coating on the gold NPs alone may not have the toxic effect on the plant.24 Therefore, it may be concluded that there may be other contributing factors except the impact of the capping agent alone.
Various chromosomal aberrations like laggard chromosomes, clumped chromosome, disturbed metaphase, sticky anaphase, diagonal anaphase, chromosomal loss, chromosomal bridge, chromosomal break, and c-mitosis were observed under the optical microscope upon the interaction of root tip cells with Au15, Au30, and Au40 at various concentrations. Aberration features like C-metaphase and diagonal anaphase (Fig. 2A1–A2) were observed upon the exposure to 10 μg mL−1 of Au15, whereas clumped metaphase and sticky anaphase (Fig. 2B1–B2) were noted in the root tip cells exposed to 1 μg mL−1 of Au15. The formation of chromosomal bridge and laggard chromosomes (Fig. 2C1–C2) was observed after the treatment with 0.1 μg mL−1 of Au15. Similarly, exposure to 10 μg mL−1 of Au30 resulted in clumped chromosome, apoptosis, and c-mitosis (Fig. 3A1–A2). Laggard metaphase and anaphase and multipolar anaphase (Fig. 3B1–B2) were noted in root tip cells after treatment with 1 μg mL−1 of Au30. Upon the exposure to 0.1 μg mL−1 of Au30, aberrations like chromosomal breaks along with ring formation and sticky chromosomes (Fig. 3C1–C2) were observed. At an exposure concentration of 10 μg mL−1 of Au40, the cells show abnormalities like chromosomal breaks and anaphase bridge formation (Fig. 4A1–A2). Likewise, bridge formation in anaphase and chromosomal breaks (Fig. 4B1–B2) were also noted in root tip cells after interaction with 1 μg mL−1 of Au40. Chromosomal loss and disturbed metaphase (Fig. 4C1–C2) were observed after treatment with 0.1 μg mL−1 of Au40. The increase in percentage of chromosomal aberration was directly proportional to the concentration of NPs and inversely proportional to the size of NPs.
The decrease of MI in the treated samples as compared to the control sample indicates that gold NPs may have an adverse impact on A. cepa. The obtained results were found to correlate with the findings of few previous reports on A. cepa exposure to metal oxide nanoparticles like zinc oxide, bismuth(III) oxide, titania, alumina nanoparticles, and metallic silver nanoparticles. AgNP- and ZnO NP-treated A. cepa root tip cells showed a gradual decrease in the MI upon increasing the concentration of NPs by giving rise to various chromosomal aberrations.31,32 A concentration-dependent decrease in MI was also reported for TiO2 NP- and AgNP-treated A. cepa root tip cells, and that could in turn lead to DNA damage by inducing oxidative stress.33,35 In another study, the accumulation of TiO2 and Al2O3 NPs within the cells was found to result in the generation of reactive oxygen species, which could thereby decrease the MI values.36,37
Mohandas and Grant have reported that the reduction in MI was due to the blockage of the G1 stage that leads to the suppression of DNA synthesis. The shifting of poles by the depolymerization of spindle fibers directs towards the occurrence of different chromosomal aberrations in metaphase and anaphase.48 The disturbance in spindle fibers and the improper folding of chromosomes result in the formation of sticky chromosomes and disturbed phases.49 Further, the sticky chromosome may cause the fragmentation of the chromosome and bridge formation, which ultimately leads to structural chromosomal mutation.33
The toxicity study carried out by Sabo-Attwood et al., suggested that gold NPs have the potential to enter tobacco plants (Nicotiana xanthi) through size-dependent mechanism.24 Further, they have demonstrated that the uptake, translocation, and internalization of gold NPs into plant cells could cause biotoxicity. The interaction of gold NPs with an aquatic macrophyte, Ceratophyllum demersum, studied by Ostroumov et al., shows the phytotoxicity of nanoparticles at a concentration of 6 × 10−6 M to 1.8 × 10−5 M. Gold NPs have the potential to induce changes in the stress response gene and could cause deteriorations in major cellular processes even in short-term exposure.25
The MI% and CA% for the H2O2 treated root cells was observed to be 17.92 ± 0.71 and 29.13 ± 4.71. The reduction in the MI% was noted to significantly decrease compared to the control and gold NPs treated cells which confirmed that the toxicity can be induced by the oxidative stress. The oxidative stress analysis was further carried to quantify the amount of oxidative stress generated in the treated root cells.
3.3. Oxidative stress analysis
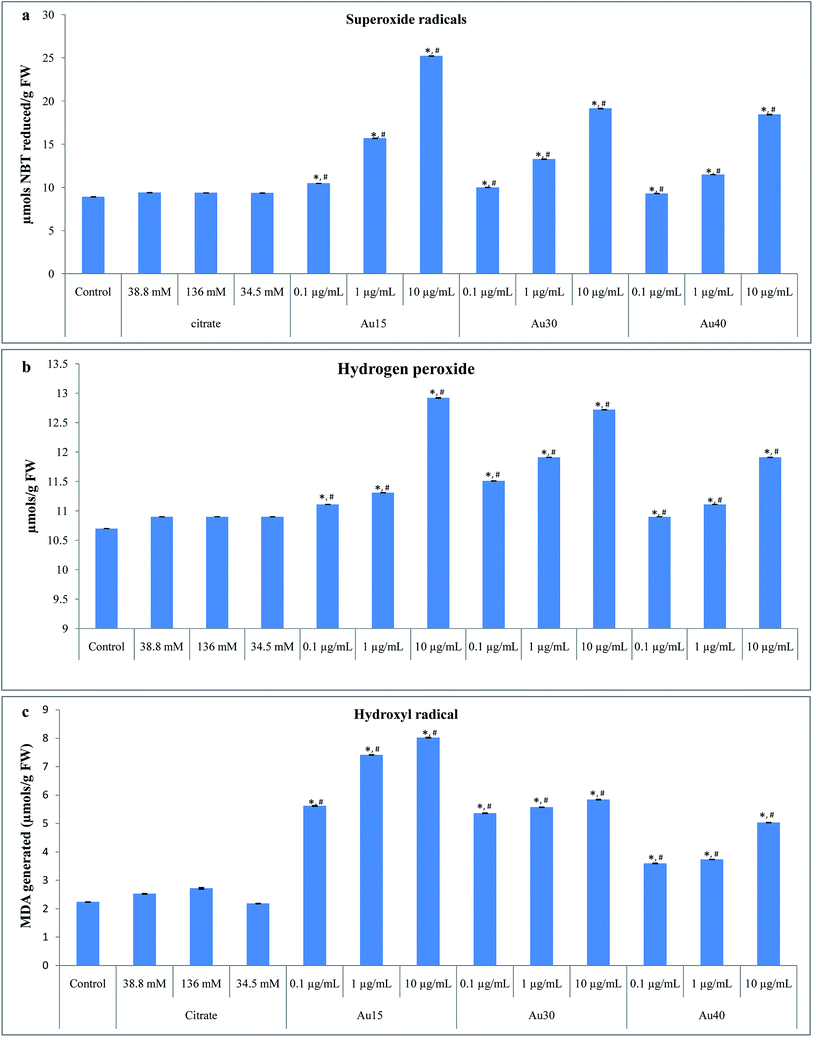
The generation of superoxide (O2˙−), hydrogen peroxide (H2O2), and hydroxyl (OH) radicals was determined in treated and untreated A. cepa root tip cells to confirm the oxidative stress induced by gold NPs. Fig. 5A shows the profile of O2˙− generation in A. cepa root tips cells before and after interaction with the different concentrations (0.1, 1, and 10 μg mL−1) of Au15, Au30, and Au40. Upon the exposure to different concentrations of Au15, the generation of O2˙− was observed to increase upon increasing the concentration of nanoparticles with respect to the control cells. Similarly, a concentration-dependent increase in O2˙− generation was also observed in Au30- and Au40-treated root tip cells. Compared to the control root tip cells, the generation O2˙− induced by Au15, Au30, and Au40 was found to be statistically significant (P < 0.001).Fig. 5B shows the H2O2 generation in A. cepa root tip cells after interaction with Au15, Au30, and Au40 at different concentrations. The H2O2 production in the gold NP-treated A. cepa root tip cells was observed to be directly proportional to the concentration of nanoparticles at different size levels. The increase in H2O2 generation was found to be statistically significant (P < 0.001) with respect to the control root tip cells.
The exposure of root tip cells to Au15 results in the production of hydroxyl radical, and it was noted to increase upon increasing the concentration of nanoparticles with respect to the control (P < 0.001). A similar effect was observed for Au30- and Au40-treated root tip cells. The determined ˙OH radical generation in A. cepa root tip cells after interaction with various concentrations of Au15, Au30, and Au40 is shown in Fig. 5C.
The generation of reactive oxygen species, O2˙−, H2O2, and ˙OH radicals in gold NP-treated root tip cells was observed to decrease with increasing sizes of NPs. The generation of free radicals was found to be enhanced for lower sized particles as compared to higher sized particles at all concentrations (0.1, 1, and 10 μg mL−1) of NPs. The increase in the generation of reactive oxygen species (O2˙−, H2O2, and ˙OH radicals) was observed to be significant (P < 0.001) as the size of nanoparticles decreased.
To ensure the effect of citrate, the generation of reactive oxygen species, O2˙−, H2O2, and ˙OH radicals was evaluated for the citrate (38.8, 136 and 34.5 mM) treated root cells. The production of reactive oxygen species in the citrate treated cells was observed to be statistically insignificant in comparison to the control root cells. Whereas, a significant increase in the generation of reactive oxygen species was noted in the gold NPs treated root cells for all concentrations in comparison to the citrate concentration employed in their synthesis. Thus, these results confirm that the gold NPs have a major impact on the generation of reactive oxygen species along with the citrate capping.
The oxidative analysis was considered as an essential mechanism to induce toxicity in cells. Oxidative stress can be influenced by various factors such as active redox cycling on the surface of NPs, oxidative groups functionalized on the NPs, and nanoparticle–cell interaction.50 Nanoparticles have the ability to enter the cell membrane and accumulate in the cytoplasm; further, this leads to mitochondrial dysfunction, oxidative stress, and cell death.
Zhai et al., have reported that gold NPs can transport through the plasmodesmata and can get accumulated on the plant cell wall. Due to narrow channels in cells, the gold NPs get aggregated. The aggregated gold NPs in cells may control the transport of nutrients and other materials from companion cells, which leads to toxic effects.51 Thus, the change in physicochemical properties of nanoparticles within the cells may pave way for ROS generation. There are prior reports on the size-dependent uptake of gold NPs that leads to toxicity and cell death in the tobacco plant.24
3.4. Lipid peroxidation
The most prominent symptoms of oxidative stress generation in biological membrane can be observed due to the peroxidation of lipids. Fig. 6 shows the gold NP-induced lipid peroxidation in A. cepa root tip cells. The malondialdehyde (MDA) content in the untreated root samples was 1.28 μM. The concentrations of MDA generated in A. cepa root tip cells upon the exposure to 0.1, 1, and 10 μg mL−1 of Au15 were found to be 1.88, 2.70, and 3.11 μM. Similarly, the stimulated MDA concentrations were observed to be 1.36, 1.80, and 2.11 μM in root tip cells after treatment with 0.1, 1, and 10 μg mL−1 of Au30. However, after exposure to 0.1, 1, and 10 μg mL−1 of Au40, 1.36, 1.37, and 1.51 μM of MDA were generated in the root cells. The MDA content in the 38.8, 136 and 34.5 mM citrate treated cells was 1.03, 1.11 and 1.05 μM. The MDA content in the citrate treated cells were found statistically insignificant with respect to control cells.The lipid peroxidation assay shows a concentration-dependent increase in the MDA level in the root tip cells after exposure to Au15, Au30, and Au40. For all the three size groups employed, the increase in the concentration of MDA was observed to be statistically significant (P < 0.001) for all the concentrations (0.1, 1, and 10 μg mL−1) of NPs with respect to control and citrate treated root cells. When comparing the effect of different sizes of gold NPs, the level of MDA in the treated root tip cells was noted to decrease significantly (P < 0.001) with increasing sizes of nanoparticles. The results obtained from the lipid peroxidation assay were in correlation with the results from the MI and oxidative stress assays.
In plant cells, the DNA damage may occur directly or indirectly by means of abiotic stress induced by nanoparticles, including heavy metals. The protonation of superoxide (O2) could produce hydroperoxyl radicals (˙OH, H2O2) in plant cells.52 Thus, the fatty acid can get converted into toxic lipid peroxides by the hydroperoxyl radical, and thereby, destroy the biological membranes.31 The release of reactive oxygen species causes thiobarbituric acid reactive species formation and enhances membrane permeability. Khan et al. have suggested that 10 nm gold NPs can produce lipid peroxidation in rat liver.53 A similar effect was observed in A. cepa root tip cells after exposure to TiO2 NPs and AgNPs.31,35
4. Conclusion
The toxicity of three different sizes of gold NPs was assessed in the plant system using A. cepa bioassay. The gold NP-treated root tip cells indicated a dose-dependent increase in chromosomal aberration and decrease in mitotic index. The mitotic index was noted to decrease for all the concentrations as the size of nanoparticles decreased. Different types of chromosomal aberrations observed in treated root tips confirmed the adverse effects of gold NPs. The interaction of A. cepa root cells with gold NPs resulted in the generation of reactive oxygen species, which was found to have a strong dependence on the dose and size of particles. These results were found to correlate well with the enhanced lipid peroxidation. Thus, the oxidative stress generation and MDA formation could be contributing factors for causing aberrations in the chromosomes of A. cepa root tips, showing the potential toxic effect of citrate-capped gold NPs. The citrate capping on the gold NPs alone had no significant effect on the mitotic index and reactive oxygen species.Acknowledgements
We would like to thank the Sophisticated Analytical Instrument Facility (SAIF) at Indian Institute of Technology, Madras for the ICP-OES analysis facility and Indian Institute of Sciences, Bangalore for the Transmission Electron Microscopy facility.References
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18–H40 CrossRef CAS PubMed.
- X. Chen, C. Li, M. Grätzel, R. Kostecki and S. S. Mao, Chem. Soc. Rev., 2012, 41, 7909–7937 RSC.
- M. Hua, S. Zhang, B. Pan, W. Zhang, L. Lv and Q. Zhang, J. Hazard. Mater., 2012, 211, 317–331 CrossRef PubMed.
- J. L. West and N. J. Halas, Annu. Rev. Biomed. Eng., 2003, 5, 285–292 CrossRef CAS PubMed.
- W.-x. Zhang, J. Nanopart. Res., 2003, 5, 323–332 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- B. A. Magnuson, T. S. Jonaitis and J. W. Card, J. Food Sci., 2011, 76, R126–R133 CrossRef CAS PubMed.
- K.-T. Yong, M. T. Swihart, H. Ding and P. N. Prasad, Plasmonics, 2009, 4, 79–93 CrossRef CAS.
- G. F. Paciotti, L. Myer, D. Weinreich, D. Goia, N. Pavel, R. E. McLaughlin and L. Tamarkin, Drug Delivery, 2004, 11, 169–183 CrossRef CAS PubMed.
- L. C. Kennedy, L. R. Bickford, N. A. Lewinski, A. J. Coughlin, Y. Hu, E. S. Day, J. L. West and R. A. Drezek, Small, 2011, 7, 169–183 CrossRef CAS PubMed.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- F. Torney, B. G. Trewyn, V. S.-Y. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS PubMed.
- R. Behra, B. Wagner, L. Sgier and D. Kistler, Aquat. Geochem., 2015, 1–12 Search PubMed.
- A. García, L. Delgado, J. A. Torà, E. Casals, E. González, V. Puntes, X. Font, J. Carrera and A. Sánchez, J. Hazard. Mater., 2012, 199, 64–72 CrossRef PubMed.
- A. Albanese and W. C. Chan, ACS Nano, 2011, 5, 5478–5489 CrossRef CAS PubMed.
- M. Schulz, L. Ma-Hock, S. Brill, V. Strauss, S. Treumann, S. Gröters, B. van Ravenzwaay and R. Landsiedel, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2012, 745, 51–57 CrossRef CAS PubMed.
- M. Larguinho, D. Correia, M. S. Diniz and P. V. Baptista, J. Nanopart. Res., 2014, 16, 1–11 CrossRef CAS.
- J. Hawthorne, C. Musante, S. K. Sinha and J. C. White, Int. J. Phytorem., 2012, 14, 429–442 CrossRef CAS PubMed.
- J. D. Judy, J. M. Unrine, W. Rao, S. Wirick and P. M. Bertsch, Environ. Sci. Technol., 2012, 46, 8467–8474 CrossRef CAS PubMed.
- Z.-J. Zhu, H. Wang, B. Yan, H. Zheng, Y. Jiang, O. R. Miranda, V. M. Rotello, B. Xing and R. W. Vachet, Environ. Sci. Technol., 2012, 46, 12391–12398 CrossRef CAS PubMed.
- J. D. Judy, J. M. Unrine and P. M. Bertsch, Environ. Sci. Technol., 2010, 45, 776–781 CrossRef PubMed.
- W. M. Lee, Y. J. An, H. Yoon and H. S. Kweon, Environ. Toxicol. Chem., 2008, 27, 1915–1921 CrossRef CAS PubMed.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS PubMed.
- T. Sabo-Attwood, J. M. Unrine, J. W. Stone, C. J. Murphy, S. Ghoshroy, D. Blom, P. M. Bertsch and L. A. Newman, Nanotoxicology, 2012, 6, 353–360 CrossRef CAS PubMed.
- B. J. Agtuca, Gold Nanoparticles in the Environment: Studying the Genetic Toxicity and Bioavailability in Soils and Hydroponic Exposures with Lycopersicon esculentum (Tomato ‘Brandywine’), 2014 Search PubMed.
- V. Kumar, P. Guleria, V. Kumar and S. K. Yadav, Sci. Total Environ., 2013, 461, 462–468 CrossRef PubMed.
- S. Arora, P. Sharma, S. Kumar, R. Nayan, P. Khanna and M. Zaidi, Plant Growth Regul., 2012, 66, 303–310 CrossRef CAS.
- S. B. Tedesco and H. D. Laughinghouse IV, Bioindicator of genotoxicity: the Allium cepa test, Intech Open Access Publisher, 2012 Search PubMed.
- W. F. Grant, Mutat. Res., Rev. Genet. Toxicol., 1982, 99, 273–291 CrossRef CAS.
- W. H. Organization, 1985.
- M. Kumari, S. S. Khan, S. Pakrashi, A. Mukherjee and N. Chandrasekaran, J. Hazard. Mater., 2011, 190, 613–621 CrossRef CAS PubMed.
- M. Kumari, A. Mukherjee and N. Chandrasekaran, Sci. Total Environ., 2009, 407, 5243–5246 CrossRef CAS PubMed.
- K. K. Panda, V. M. M. Achary, R. Krishnaveni, B. K. Padhi, S. N. Sarangi, S. N. Sahu and B. B. Panda, Toxicol. in Vitro, 2011, 25, 1097–1105 CrossRef CAS PubMed.
- R. Liman, Chemosphere, 2013, 93, 269–273 CrossRef CAS PubMed.
- M. Ghosh, M. Bandyopadhyay and A. Mukherjee, Chemosphere, 2010, 81, 1253–1262 CrossRef CAS PubMed.
- S. Pakrashi, N. Jain, S. Dalai, J. Jayakumar, P. T. Chandrasekaran, A. M. Raichur, N. Chandrasekaran and A. Mukherjee, PLoS One, 2014, 9, e87789 Search PubMed.
- A. Rajeshwari, S. Kavitha, S. A. Alex, D. Kumar, A. Mukherjee, N. Chandrasekaran and A. Mukherjee, Environ. Sci. Pollut. Res., 2015, 1–10 Search PubMed.
- D. Kumar, A. Rajeshwari, P. S. Jadon, G. Chaudhuri, A. Mukherjee, N. Chandrasekaran and A. Mukherjee, J. Environ. Sci., 2015, 38, 150–157 CrossRef PubMed.
- G. Frens, Nature, 1973, 241, 20–22 CAS.
- S. K. Sivaraman, S. Kumar and V. Santhanam, J. Colloid Interface Sci., 2011, 361, 543–547 CrossRef CAS PubMed.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- A. Bakare, A. Mosuro and O. Osibanjo, J. Environ. Biol., 2000, 21, 263–271 CAS.
- A. Kiba, C. Miyake, K. Toyoda, Y. Ichinose, T. Yamada and T. Shiraishi, Phytopathology, 1997, 87, 846–852 CrossRef CAS PubMed.
- F. Loreto and V. Velikova, Plant Physiol., 2001, 127, 1781–1787 CrossRef CAS PubMed.
- B. Halliwell, J. M. Gutteridge and O. I. Aruoma, Anal. Biochem., 1987, 165, 215–219 CrossRef CAS PubMed.
- R. S. Dhindsa, P. Plumb-Dhindsa and T. A. Thorpe, J. Exp. Bot., 1981, 32, 93–101 CrossRef CAS.
- S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407–10410 CrossRef CAS.
- C. Darlington and J. McLeish, 1951.
- M. d. G. Medeiros and C. S. Takahashi, Cytologia, 1987, 52, 255–259 CrossRef.
- N. von Moos and V. I. Slaveykova, Nanotoxicology, 2014, 8, 605–630 CrossRef CAS PubMed.
- G. Zhai, K. S. Walters, D. W. Peate, P. J. Alvarez and J. L. Schnoor, Environ. Sci. Technol. Lett., 2014, 1, 146–151 CrossRef CAS PubMed.
- H. Zhang, Y. Jiang, Z. He and M. Ma, J. Plant Physiol., 2005, 162, 977–984 CrossRef CAS PubMed.
- H. A. Khan, M. A. K. Abdelhalim, M. S. Al-Ayed and A. S. Alhomida, Saudi J. Biol. Sci., 2012, 19, 461–464 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04712b |
| This journal is © The Royal Society of Chemistry 2016 |