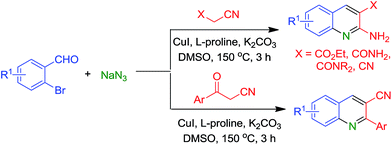
Copper-catalyzed synthesis of quinoline derivatives via tandem Knoevenagel condensation, amination and cyclization†‡
Shiv Dhiman,
Hitesh Kumar Saini,
Nitesh Kumar Nandwana,
Dalip Kumar and
Anil Kumar*
Department of Chemistry, Birla Institute of Technology and Science, Pilani, 333031 Rajasthan, India. E-mail: anilkumar@pilani.bits-pilani.ac.in
First published on 23rd February 2016
Abstract
A novel regioselective synthesis of 2-aminoquinolines and 2-arylquinoline-3-carbonitriles is described via copper-mediated tandem reaction. Formation of substituted quinolines involves Knoevenagel condensation of ortho-bromobenzaldehyde with active methylene nitriles followed by copper-catalyzed reductive amination and intramolecular cyclization.
Introduction
The quinoline skeleton is one of the most prevalent motifs, found in many drugs, natural products and pharmacologically active substances.1 Compounds with this motif have been found to possess a broad range of biological activities such as anticancer,2 antifungal,3 antimalarial,4 antituberculosis,5 antiprotozoal,6 antiinflammatory,7 antineoplastic,8 and inhibition of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (HER-2) kinases.9 Functionalized quinolines are also used as dyestuffs,10 asymmetric catalysts,11 ligands for transition metal complexes and for the preparation of nano- and mesostructures with improved electronic and photonic properties.12 Some promising compounds with quinoline motif are shown in Fig. 1.Generally, quinoline and its derivatives are prepared by conventional methods including Friedländer, Pfitzinger, Doebner–von Miller, Skraup, Combes and Conrad–Limpach reactions.13 In past, these reactions have been successfully employed for quinoline synthesis but they require stoichiometric amounts of acidic reagents and are often performed under harsh conditions. Moreover, use of harsh reaction conditions and highly reactive acid catalysts restrict involvement of functionally substituted substrates for synthesis of corresponding quinolines. In recent years, several approaches based on tandem reactions, transition metal catalysts and alternative starting materials have been developed for mild and efficient syntheses of quinolines.14 For example, Li et al. have developed a copper-catalyzed synthesis of quinolines from ortho-acylanilines and alkenyl iodides.15 Yu and co-workers developed synthesis of quinolines from 2-aminobenzylamine and ketones via copper-catalyzed C–N bond cleavage.16 Consisting CuI and a secondary amine, Patil group developed a co-operative catalytic system for the synthesis of 2-substituted quinolines by utilizing 2-aminobenzaldehydes and terminal alkynes.17 In particular, 2-aminoquinolines are important target molecules because of their sub-nanomolar potency for BACE inhibition18 and selective neuronal nitric oxide synthase (nNOS) inhibition activities.19 Only a handful reports are available for synthesis of 2-aminoquinoline derivatives.20 Tomioka et al. have reported one-pot synthesis of 2-aminoquinolines from 2-nitrobenzaldehydes and acetonitrile via stereoselective olefination followed by reductive cyclization.20a Jiang group have synthesized 2-aminoquinolines by Pd-catalyzed reactions of gem-dibromovinylanilines and tert-butyl isocyanide.20b,20c Liu et al. have utilized 4-halo-2-aminoquinolines in a Pd-catalyzed intermolecular aerobic oxidative cyclization of 2-ethynylanilines with isocyanide to prepare 2-aminoquinolines.20d Owing to potential applications in various fields, quinolines continue to attract the attention of scientists from different areas, and the development of new synthetic methods for quinolines are still of great interest.
Furthermore, the direct amination of aryl halides using NaN3 or TMSN3 as the amino source in the presence of copper or copper salt has been reported.21 Trapping of the intermediate azide or amine of this copper catalyzed reductive amination have resulted in the synthesis of bioactive heterocycles such as quinolones, 3-aminoquinolines, 3-aminocoumarines, tetracyclic indoloindol-3-ones, pseudo-indoxyl derivatives, indazole and 2-aroylindoles.22 As a part of our continuous efforts in the development of new synthetic methods for heterocyclic compounds by employing C–C/C–N coupling reactions,23 herein we report our results on one-pot copper-catalyzed regioselective synthesis of 2-aminoquinolines and 2-arylquinoline-3-carbonitriles via tandem reactions involving 2-bromobenzaldehydes, active methylene nitriles and sodium azide (Scheme 1).
Results and discussion
Our initial study began with the reaction of 2-bromobenzaldehyde (1a) with ethyl cyanoacetate (2a) and sodium azide in the presence of CuI (10 mol%), L-proline (20 mol%), K2CO3 (2.5 equiv.) in N,N′-dimethylformamide (DMF) at 150 °C under air atmosphere for 3 h. Gratifyingly, ethyl 2-aminoquinoline-3-carboxylate (4aa) was isolated in 35% yield (entry 1). The structure of 4aa was characterized by various spectroscopic techniques such as IR, NMR and mass spectrometry. In IR spectrum of 4aa, strong peaks at 3410 and 1697 cm−1 are indicative of –NH2 and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities, respectively. In 1H NMR spectrum, characteristic singlets at δ 8.77 and δ 7.26 (broad) for C4–H and NH2 protons were observed. The carbonyl carbon of CO2Et appeared at δ 166.5 along with all other expected peaks in 13C NMR spectrum of 4aa. Further, the structure was ascertained by HRMS analysis of 4aa which showed a molecular ion C12H13N2O2+ [M + H]+ peak at m/z 217.0976 in agreement with the calculated mass 217.0972.
O functionalities, respectively. In 1H NMR spectrum, characteristic singlets at δ 8.77 and δ 7.26 (broad) for C4–H and NH2 protons were observed. The carbonyl carbon of CO2Et appeared at δ 166.5 along with all other expected peaks in 13C NMR spectrum of 4aa. Further, the structure was ascertained by HRMS analysis of 4aa which showed a molecular ion C12H13N2O2+ [M + H]+ peak at m/z 217.0976 in agreement with the calculated mass 217.0972.
To further improve the yield of tandem product 4aa, various experimental conditions were screened by varying copper catalysts, ligands, bases and solvents (Table 1). Firstly, screening of various copper salts such as CuI, CuCl2, CuBr, Cu(OAc)2·H2O, CuOTf and CuSO4 revealed that CuI was the best catalyst for this transformation giving highest yield of 4aa (Table 1, entries 1–6). Further investigations on the effect of catalyst loading suggested that 20 mol% CuI afforded 4aa in highest yield (55%) (Table 1, entries 1, 7 and 8). Among various bases (K2CO3, K3PO4, tBuOK, Cs2CO3, Na2CO3, NaOMe, Et3N and DBU) examined (Table 1, entries 7, 9–16), K2CO3 was found to be the most suitable base. In case of triethylamine, the reaction exclusively led to Knoevenagel adduct (Table 1, entry 14). Reactions in different solvents namely DMSO, N,N-dimethylacetamide (DMA), toluene, N-methyl-2-pyrrolidone (NMP), 1,4-dioxane and PEG-400 (Table 1, entries 16–21) revealed DMSO as a the solvent of choice for this reaction. When the reaction was performed in toluene at 120 °C only Knoevenagel adduct was obtained in 45% yield (Table 1, entry 20). Finally, by screening of different ligands (Table 1, entries 16, 21–25), L-proline was found to be the most effective ligand. The reaction was stopped at the Knoevenagel adduct in the absence of catalyst (Table 1, entry 26) and yield of desired product 4aa was diminished in the absence of L-proline (Table 1, entry 27).
| Entry | Catalyst (mol%) | Ligand (mol%) | Base (2.5 equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.54 mmol), 2a (0.65 mmol) NaN3 (3) (0.81 mmol), catalyst, ligand, base (2.5 equiv.), 150 °C, air atmosphere, 3 h.b Isolated yields.c Reaction time 12 h, only Knoevenagel adduct 6.d Knoevenagel adduct 6 (120 °C, 45%).e 1,10-Phen = 1,10-phenanthroline.f 8-HQ = 8-hydroxyquinoline. | |||||
| 1 | CuI (10) | L-Proline (20) | K2CO3 | DMF | 35 |
| 2 | CuCl2 (10) | L-Proline (20) | K2CO3 | DMF | 9 |
| 3 | CuBr (10) | L-Proline (20) | K2CO3 | DMF | 20 |
| 4 | Cu(OAc)2·H2O (10) | L-Proline (20) | K2CO3 | DMF | 13 |
| 5 | CuOTf (10) | L-Proline (20) | K2CO3 | DMF | 31 |
| 6 | CuSO4 (10) | L-Proline (20) | K2CO3 | DMF | 26 |
| 7 | CuI (20) | L-Proline (40) | K2CO3 | DMF | 55 |
| 8 | CuI (30) | L-Proline (60) | K2CO3 | DMF | 50 |
| 9 | CuI (20) | L-Proline (40) | K3PO4 | DMF | 38 |
| 10 | CuI (20) | L-Proline (40) | tBuOK | DMF | 30 |
| 11 | CuI (20) | L-Proline (40) | CsCO3 | DMF | 10 |
| 12 | CuI (20) | L-Proline (40) | Na2CO3 | DMF | 8 |
| 13 | CuI (20) | L-Proline (40) | NaOMe | DMF | 11 |
| 14 | CuI (20) | L-Proline (40) | Et3N | DMF | —c |
| 15 | CuI (20) | L-Proline (40) | DBU | DMF | 6 |
| 16 | CuI (20) | L-Proline (40) | K2CO3 | DMSO | 62 |
| 17 | CuI (20) | L-Proline (40) | K2CO3 | NMP | 28 |
| 18 | CuI (20) | L-Proline (40) | K2CO3 | DMA | 37 |
| 19 | CuI (20) | L-Proline (40) | K2CO3 | Dioxane | 10 |
| 20 | CuI (20) | L-Proline (40) | K2CO3 | Toluene | —d |
| 21 | CuI (20) | L-Proline (40) | K2CO3 | PEG-400 | — |
| 22 | CuI (20) | Glycine (40) | K2CO3 | DMSO | 38 |
| 23 | CuI (20) | DMEDA (40) | K2CO3 | DMSO | 42 |
| 24 | CuI (20) | 1,10-Phene (40) | K2CO3 | DMSO | 12 |
| 25 | CuI (20) | 8-HQf (40) | K2CO3 | DMSO | 9 |
| 26 | — | L-Proline (40) | K2CO3 | DMSO | —c |
| 27 | CuI (20) | — | K2CO3 | DMSO | 20 |
With the optimized reaction condition in hand (Table 1, entry 16), we explored the substrate generality for this tandem reaction by employing the substituted 2-bromobenzaldehydes and active methylene nitriles (Table 2). Reactions of substituted 2-bromobenzaldehydes 1a–c with different active methylene nitriles 2a–e gave corresponding 2-aminoquinolines in moderate to good yields (4aa–ae). The method tolerated different functional groups such as cyano, methoxy, ester, and amide. Structures of all the compounds were confirmed by IR, NMR (1H & 13C) and HRMS data (ESI‡).
Notably, when benzoylacetonitrile (X = ArCO, 3) was used as an active methylene nitrile, instead of expected 2-aminoquinoline, 2-phenylquinoline-3-carbonitrile (5aa) was obtained in 59% yield. Formation of 5aa prompted us to evaluate the regioselectivity of this reaction for benzoylacetonitriles. As can be seen from Table 2, benzoylacetonitriles containing methyl, methoxy, dioxole and chloro substituent reacted efficiently with 2-bromobenzaldehydes to give corresponding quinoline-3-carbonitriles (5aa–ah) in moderate to good yields. Similarly, 2-bromobenzaldehydes bearing methoxy and chloro groups were treated with different substituted benzoylacetonitriles to give corresponding 2-arylquinoline-3-carbonitriles in moderate to good yields (5ba–ch).
Control experiments were performed to evaluate the possible reaction pathway for the tandem sequences to produce 4aa and 5aa (Scheme 2). Initially, 2-bromobenzaldehyde (1a) was reacted with ethyl cyanoacetate using K2CO3 in DMSO at room temperature for 30 min, only Knoevenagel adduct 6 was obtained in 78% yield (Scheme 2A). When adduct 6, NaN3, CuI, L-proline and K2CO3 were heated at 150 °C for 2 h, only the desired product 4aa was isolated in 70% yield (Scheme 2B). Exclusive formation of 4aa can be due to the relative reactivity of cyano group over ester in 6. Reaction of 1a with NaN3, CuI, L-proline and K2CO3 at 120 °C gave 2-aminobenzaldehyde (8) in 64% yield (Scheme 2C). However, treatment of 1a with ethyl cyanoacetate in the presence of NaN3, CuI, L-proline and K2CO3 at room temperature exclusively resulted in adduct 6. When the same reaction mixture was heated at 100 °C, ethyl 3-(2-aminophenyl)-2-cyanoacrylate (9) was obtained in 50% yield along with the desired product 4aa in 8% yield (Scheme 2D). Further, when 9 was heated at 150 °C in the presence of K2CO3 in DMSO, 4aa was obtained in 33% yield after 5 h, and 9 was recovered in 46% yield (Scheme 2E). From these control experiments, we concluded that ethyl 3-(2-bromophenyl)-2-cyanoacrylate (6) and ethyl 3-(2-aminophenyl)-2-cyanoacrylate (9) are key intermediates for the formation of 2-aminoquinoline. Similarly, reaction of 1a with 3a in absence of copper catalyst gave 2-benzoyl-3-(2-bromophenyl)acrylonitrile (10) which on reaction with NaN3, CuI, L-proline and K2CO3 at 150 °C after 2 h resulted in exclusive formation of 5aa (Scheme 2F and G). This may be attributed to the relative electrophilicity of carbonyl group over nitrile group. Structure of 10 was confirmed NMR, mass and single X-ray analysis (CCDC 1433055, Fig. 2).
 | ||
| Fig. 2 ORTEP diagram (with 35% ellipsoid probability) for 10 (CCDC 1433055). | ||
On the basis of control experiments and literature reports,22a,24 a possible mechanism for the copper-catalyzed tandem reaction has been described (Scheme 3). Initially, the reaction of 2-brombenzaldehye and ethyl cyanoacetate generated Knoevenagel adduct 6. Reductive amination of 6 using sodium azide in the presence of copper catalyst produced 2-(2-aminobenzylidene)malononitrile (9). This is in accordance with earlier reports wherein sodium azide has been used as ammonia surrogate to prepare primary amines and nitrogen containing heterocycles in a copper catalyzed reductive amination of aryl halides.14f,22c,22d Subsequently, intramolecular cyclization of 9 led to the formation of ethyl 2-aminoquinoline-3-carboxylate (4) via nucleophilic attack of amine onto nitrile followed by tautomerization. In case of benzoylacetonitrile, intermediate 10 formed after Knoevenagel condensation underwent reductive amination followed by intramolecular condensation to afford 2-arylquinoline-3-carbonitrile (5).
Next, the synthetic worth of the developed methodology was demonstrated by one-pot synthesis of pyrimido[4,5-b]quinolin-4(3H)-one derivatives (12a–c). In all the cases, reactions underwent smooth conversion to afford the corresponding pyrimido[4,5-b]quinolin-4(3H)-ones 12a–c in moderate to good (43–54%) yields (Scheme 4).
Conclusions
In conclusion, we have successfully developed an efficient and straightforward copper-catalyzed regioselective synthesis of 2-aminoquinolines and 2-arylquinoline-3-carbonitriles from readily available 2-bromobenzaldehydes, active methylene nitriles and sodium azide. The developed three-component, one-pot tandem protocol displays broad substrate scope, good functional group tolerance and gives quinolines in moderate to good yields. The developed methodology can further be utilized for one-pot synthesis of pyrimido[4,5-b]quinolin-4(3H)-ones.Experimental section
Melting points were determined in open capillary tubes on an automated melting point apparatus and are uncorrected. Reactions were monitored by using thin layer chromatography (TLC) on 0.2 mm silica gel F254 plates. The chemical structures of final products were determined by their NMR spectra (1H and 13C NMR). Chemical shifts are reported in parts per million (ppm) using deuterated solvent peak or tetramethylsilane as an internal standard. The HRMS data were recorded on a mass spectrometer with electrospray ionization and TOF mass analyzer. Some of benzoyl acetonitriles and 2-cyano acetamides were synthesized according to published procedure.25 All other chemicals were obtained from the commercial suppliers and used without further purification.Representative procedure for synthesis of 2-aminoquinolines (4)
A mixture of 2-bromobenzaldehyde (100 mg, 0.54 mmol), ethyl cyanoacetate (73 mg. 0.65 mmol), sodium azide (52 mg, 0.81 mmol), CuI (20 mol%), L-proline (40 mol%) and K2CO3 (186 mg, 2.5 equiv.) in DMSO (2 mL) was mixed under air atmosphere at room temperature and then heated to 150 °C for 3 h. After cooling to ambient temperature, the reaction mass was quenched with ice-cold aqueous solution of NH4Cl (30 mL), filtered through a bed of celite and the plug washed with ethyl acetate (20 mL). The resulting filtrate was extracted with ethyl acetate (2 × 20 mL) and the combined organic layers dried over anhydrous Na2SO4 and concentrated under reduced pressure. Desired product 4aa (72 mg, 62%) was isolated by column chromatography on silica gel (100–200 mesh) using ethyl acetate/hexane (30%, v/v) as eluant.Representative procedure for synthesis of 2-arylquinoline-3-carbonitriles (5)
A mixture of 2-bromobenzaldehyde (100 mg, 0.54 mmol), benzoylacetonitriles (94 mg, 0.65 mmol), sodium azide (52 mg, 0.81 mmol), CuI (20 mol%), L-proline (40 mol%) and K2CO3 (186 mg, 2.5 equiv.) in DMSO (2 mL) was mixed under air atmosphere at room temperature and then heated to 150 °C for 3 h. After cooling to ambient temperature, the reaction mass was quenched with ice-cold aqueous solution of NH4Cl (30 mL), filtered through a bed of celite and the plug washed with ethyl acetate (20 mL). The resulting filtrate was extracted with ethyl acetate (2 × 20 mL) and the combined organic layers dried over anhydrous Na2SO4 and concentrated under reduced pressure. Desired product 5aa (73 mg, 59%) was isolated by column chromatography on silica gel (100–200 mesh) using ethyl acetate/hexane (10%, v/v) as eluant.Ethyl 3-(2-bromophenyl)-2-cyanoacrylate (6)
Colorless oil, 78%; 1H NMR (400 MHz, CDCl3) δ 8.63 (s, 1H), 8.17 (dd, J = 7.8, 1.6 Hz, 1H), 7.71 (dd, J = 8.0, 1.2 Hz, 1H), 7.46 (td, J = 7.4, 0.8 Hz, 1H), 7.38 (td, J = 7.7, 1.7 Hz, 1H), 4.41 (q, J = 7.1 Hz, 1H), 1.41 (t, J = 7.1 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 161.8, 153.9, 133.7, 133.6, 131.7, 130.1, 128.1, 126.6, 114.7, 106.4, 63.0, 14.2; HRMS for C12H11BrNO2 [M + H+] calcd 279.9968, found 279.9973 and 281.9954 [M + 2 + H+].2-Aminobenzaldehyde (8)
Pale yellow liquid; 42 mg (64%); 1H NMR (400 MHz, DMSO-d6) δ 9.81 (d, J = 0.4 Hz, 1H), 7.53 (dd, J = 7.8, 1.6 Hz, 1H), 7.30 (ddd, J = 8.5, 7.0, 1.7 Hz, 1H), 7.12 (bs, 2H), 6.76 (d, J = 8.4 Hz, 1H), 6.64 (ddd, J = 7.9, 7.0, 1.0 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 194.5, 151.2, 136.0, 135.5, 118.2, 116.3, 115.4; HRMS for C7H8NO [M + H+] calcd 122.0600, found 121.0581.Ethyl 3-(2-aminophenyl)-2-cyanoacrylate (9)
Brown solid; 58 mg (50%); mp 123–125 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.55 (s, 1H), 8.32 (d, J = 8.6 Hz, 1H), 8.09 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 8.2 Hz, 3H), 7.47 (t, J = 7.4 Hz, 1H), 4.42 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.50, 147.65, 141.08, 133.74, 130.93, 130.68, 125.00, 120.86, 117.26, 110.72, 62.23, 14.54; 1H NMR (400 MHz, DMSO-d6, D2O exchange) δ 8.58 (s, 1H), 8.26 (d, J = 8.5 Hz, 1H), 8.04 (d, J = 7.7 Hz, 1H), 7.85 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.3 Hz, 1H), 4.39 (q, J = 6.9 Hz, 2H), 1.37 (t, J = 7.0 Hz, 3H); HRMS for C12H13N2O2 [M + H+] calcd 217.0972, found 217.0975.2-Benzoyl-3-(2-bromophenyl)acrylonitrile (10)
Crystalline off white solid; 234 mg (70%); mp 126–128 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H), 8.25 (d, J = 7.1 Hz, 1H), 7.94 (d, J = 7.3 Hz, 2H), 7.73 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.4 Hz, 1H), 7.57 (t, J = 7.6 Hz, 2H), 7.51 (t, J = 7.6 Hz, 1H), 7.42 (t, J = 7.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 188.8, 154.1, 135.3, 133.8, 133.7, 133.6, 132.1, 130.10, 129.6, 128.8, 128.2, 126.5, 115.7, 113.6; HRMS for C16H11BrNO [M + H+] calcd 312.0019, found 312.0015.Ethyl 3-(2-azidophenyl)-2-cyanoacrylate (11)
1H NMR (400 MHz, CDCl3) δ 8.79 (s, 1H), 8.78 (d, J = 9.0 Hz, 1H), 8.14 (dd, J = 8.1, 1.1 Hz, 1H), 8.04 (td, J = 8.4, 7.9, 1.3 Hz, 1H), 7.85–7.80 (m, 1H), 4.64 (q, J = 7.1 Hz, 2H), 1.55 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6, 145.7, 138.1, 133.6, 132.0, 130.5, 128.6, 122.7, 117.2, 117.1, 62.7, 14.4. MS (ESI) m/z calcd for C12H11N4O2 [M + H+] 243.09, found 243.15.Representative procedure for synthesis of pyrimido[4,5-b]quinolin-4(3H)-ones (12)
A mixture of 2-bromobenzaldehyde (100 mg, 0.54 mmol), 2-cyanoacetamide (54 mg, 0.65 mmol), sodium azide (52 mg, 0.81 mmol), CuI (20 mol%), L-proline (40 mol%) and K2CO3 (186 mg, 2.5 equiv.) in DMSO (2 mL) was mixed under air atmosphere at room temperature and then heated to 150 °C for 3 h. After cooling to ambient temperature, benzaldehyde (69 mg, 0.65 mmol) was added and reaction mixture was then again heated to 150 °C for 2 h. After cooling to ambient temperature, the reaction mass was quenched with ice-cold aqueous solution of NH4Cl (30 mL), filtered through a bed of celite and the plug washed with ethyl acetate (20 mL). The resulting filtrate was extracted with ethyl acetate (2 × 20 mL) and the combined organic layers dried over anhydrous Na2SO4 and concentrated under reduced pressure. Desired product 12a (77 mg, 52%) was isolated by column chromatography on silica gel (100–200 mesh) using ethyl acetate/hexane (30%, v/v) as eluent.Acknowledgements
We sincerely acknowledge financial support from Ranbaxy Laboratories Pvt. Ltd. (India) to carry out this work. HKS is grateful to CSIR, New Delhi, India for senior research fellowship. Authors thank Prof. Alaknanda Hajra for his help in recording and analyzing single-crystal X-ray diffraction data for intermediate 10 and Miss Pinku Kaswan for her kind suggestions and discussion.Notes and references
- (a) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166–187 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2007, 24, 223–246 RSC.
- (a) S. H. Chan, C. H. Chui, S. W. Chan, S. H. L. Kok, D. Chan, M. Y. T. Tsoi, P. H. M. Leung, A. K. Y. Lam, A. S. C. Chan, K. H. Lam and J. C. O. Tang, ACS Med. Chem. Lett., 2013, 4, 170–174 CrossRef CAS PubMed; (b) O. Afzal, S. Kumar, M. R. Haider, M. R. Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2015, 97, 871–910 CrossRef CAS PubMed.
- (a) R. Musiol, J. Jampilek, V. Buchta, L. Silva, H. Niedbala, B. Podeszwa, A. Palka, K. Majerz-Maniecka, B. Oleksyn and J. Polanski, Bioorg. Med. Chem., 2006, 14, 3592–3598 CrossRef CAS PubMed; (b) A. Oliva, K. M. Meepagala, D. E. Wedge, D. Harries, A. L. Hale, G. Aliotta and S. O. Duke, J. Agric. Food Chem., 2003, 51, 890–896 CrossRef CAS PubMed.
- (a) V. Korotchenko, R. Sathunuru, L. Gerena, D. Caridha, Q. Li, M. Kreishman-Deitrick, P. L. Smith and A. J. Lin, J. Med. Chem., 2015, 58, 3411–3431 CrossRef CAS PubMed; (b) C. Teixeira, N. Vale, B. Pérez, A. Gomes, J. R. B. Gomes and P. Gomes, Chem. Rev., 2014, 114, 11164–11220 CrossRef CAS PubMed.
- (a) S. Eswaran, A. V. Adhikari, I. H. Chowdhury, N. K. Pal and K. D. Thomas, Eur. J. Med. Chem., 2010, 45, 3374–3383 CrossRef CAS PubMed; (b) K. D. Thomas, A. V. Adhikari, S. Telkar, I. H. Chowdhury, R. Mahmood, N. K. Pal, G. Row and E. Sumesh, Eur. J. Med. Chem., 2011, 46, 5283–5292 CrossRef CAS PubMed; (c) A. Lilienkampf, J. Mao, B. Wan, Y. Wang, S. G. Franzblau and A. P. Kozikowski, J. Med. Chem., 2009, 52, 2109–2118 CrossRef CAS PubMed.
- (a) F. Hayat, E. Moseley, A. Salahuddin, R. L. Van Zyl and A. Azam, Eur. J. Med. Chem., 2011, 46, 1897–1905 CrossRef CAS PubMed; (b) A. Salahuddin, A. Inam, R. L. van Zyl, D. C. Heslop, C.-T. Chen, F. Avecilla, S. M. Agarwal and A. Azam, Bioorg. Med. Chem., 2013, 21, 3080–3089 CrossRef CAS PubMed.
- R. de Oliveira Lopes, N. C. Romeiro, C. K. F. de Lima, L. Louback da Silva, A. L. Palhares de Miranda, P. G. B. D. Nascimento, F. Q. Cunha, E. J. Barreiro and L. M. Lima, Eur. J. Med. Chem., 2012, 54, 264–271 CrossRef PubMed.
- D. K. Heidary, B. S. Howerton and E. C. Glazer, J. Med. Chem., 2014, 57, 8936–8946 CrossRef CAS PubMed.
- H.-R. Tsou, E. G. Overbeek-Klumpers, W. A. Hallett, M. F. Reich, M. B. Floyd, B. D. Johnson, R. S. Michalak, R. Nilakantan, C. Discafani, J. Golas, S. K. Rabindran, R. Shen, X. Shi, Y.-F. Wang, J. Upeslacis and A. Wissner, J. Med. Chem., 2005, 48, 1107–1131 CrossRef CAS PubMed.
- Z. Sadeghi and J. Safari, Dyes Pigm., 2006, 70, 164–170 CrossRef CAS.
- M. M. Biddle, M. Lin and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 3830–3831 CrossRef CAS PubMed.
- (a) H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259–3302 RSC; (b) S. Tao, L. Li, J. Yu, Y. Jiang, Y. Zhou, C.-S. Lee, S.-T. Lee, X. Zhang and O. Kwon, Chem. Mater., 2009, 21, 1284–1287 CrossRef CAS; (c) S.-J. Lee, J.-S. Park, M. Song, I. A. Shin, Y.-I. Kim, J. W. Lee, J.-W. Kang, Y.-S. Gal, S. Kang, J. Y. Lee, S.-H. Jung, H.-S. Kim, M.-Y. Chae and S.-H. Jin, Adv. Funct. Mater., 2009, 19, 2205–2212 CrossRef CAS.
- G. Jones, in Chem. Heterocycl. Comp., John Wiley & Sons, Inc., 2008, pp. 93–318 Search PubMed.
- (a) S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463–24476 RSC; (b) H. Batchu, S. Bhattacharyya and S. Batra, Org. Lett., 2012, 14, 6330–6333 CrossRef CAS PubMed; (c) K. Wu, Z. Huang, C. Liu, H. Zhang and A. Lei, Chem. Commun., 2015, 51, 2286–2289 RSC; (d) M. Rehan, G. Hazra and P. Ghorai, Org. Lett., 2015, 17, 1668–1671 CrossRef CAS PubMed; (e) J. B. Bharate, R. A. Vishwakarma and S. B. Bharate, RSC Adv., 2015, 5, 42020–42053 RSC; (f) B. Li, C. Guo, X. Fan, J. Zhang and X. Zhang, Tetrahedron Lett., 2014, 55, 5944–5948 CrossRef CAS.
- L. Kong, Y. Zhou, H. Huang, Y. Yang, Y. Liu and Y. Li, J. Org. Chem., 2015, 80, 1275–1278 CrossRef CAS PubMed.
- L.-Y. Xi, R.-Y. Zhang, L. Zhang, S.-Y. Chen and X.-Q. Yu, Org. Biomol. Chem., 2015, 13, 3924–3930 CAS.
- N. T. Patil and V. S. Raut, J. Org. Chem., 2010, 75, 6961–6964 CrossRef CAS PubMed.
- Y. Cheng, T. C. Judd, M. D. Bartberger, J. Brown, K. Chen, R. T. Fremeau, D. Hickman, S. A. Hitchcock, B. Jordan, V. Li, P. Lopez, S. W. Louie, Y. Luo, K. Michelsen, T. Nixey, T. S. Powers, C. Rattan, E. A. Sickmier, D. J. S. Jean, R. C. Wahl, P. H. Wen and S. Wood, J. Med. Chem., 2011, 54, 5836–5857 CrossRef CAS PubMed.
- M. A. Cinelli, H. Li, G. Chreifi, P. Martásek, L. J. Roman, T. L. Poulos and R. B. Silverman, J. Med. Chem., 2014, 57, 1513–1530 CrossRef CAS PubMed.
- (a) T. Tomioka, Y. Takahashi and T. Maejima, Org. Biomol. Chem., 2012, 10, 5113–5118 RSC; (b) L. Hu, W. Gui, Z. Liu and B. Jiang, RSC Adv., 2014, 4, 38258–38262 RSC; (c) B. Jiang, L. Hu and W. Gui, RSC Adv., 2014, 4, 13850–13853 RSC; (d) B. Liu, H. Gao, Y. Yu, W. Wu and H. Jiang, J. Org. Chem., 2013, 78, 10319–10328 CrossRef CAS PubMed; (e) L. Zhang, L. Zheng, B. Guo and R. Hua, J. Org. Chem., 2014, 79, 11541–11548 CrossRef CAS PubMed.
- (a) J. T. Markiewicz, O. Wiest and P. Helquist, J. Org. Chem., 2010, 75, 4887–4890 CrossRef CAS PubMed; (b) S. Messaoudi, J. D. Brion and M. Alami, Mini-Rev. Med. Chem., 2011, 8, 448–454 CrossRef CAS.
- (a) T. Maejima, Y. Shimoda, K. Nozaki, S. Mori, Y. Sawama, Y. Monguchi and H. Sajiki, Tetrahedron, 2012, 68, 1712–1722 CrossRef CAS; (b) S. Messaoudi, J.-D. Brion and M. Alami, Adv. Synth. Catal., 2010, 352, 1677–1687 CrossRef CAS; (c) Y. Goriya and C. V. Ramana, Chem. Commun., 2013, 49, 6376–6378 RSC; (d) Y. Goriya and C. V. Ramana, Chem. Commun., 2014, 50, 7790–7792 RSC; (e) A. M. Kulkarni, K. Srinivas, M. V. Deshpande and C. V. Ramana, Org. Chem. Front., 2016, 3, 43–46 RSC; (f) J. Peng, Z. Xie, M. Chen, J. Wang and Q. Zhu, Org. Lett., 2014, 16, 4702–4705 CrossRef CAS PubMed.
- (a) N. K. Nandwana, K. Pericherla, P. Kaswan and A. Kumar, Org. Biomol. Chem., 2015, 13, 2947–2950 RSC; (b) S. Dhiman, K. Pericherla, N. K. Nandwana, D. Kumar and A. Kumar, J. Org. Chem., 2014, 79, 7399–7404 CrossRef CAS PubMed; (c) K. Pericherla, P. Khedar, B. Khungar and A. Kumar, Chem. Commun., 2013, 49, 2924–2926 RSC; (d) K. Pericherla, A. Jha, B. Khungar and A. Kumar, Org. Lett., 2013, 15, 4304–4307 CrossRef CAS PubMed.
- (a) Y. Kim, M. R. Kumar, N. Park, Y. Heo and S. Lee, J. Org. Chem., 2011, 76, 9577–9583 CrossRef CAS PubMed; (b) D. Dhar and W. B. Tolman, J. Am. Chem. Soc., 2015, 137, 1322–1329 CrossRef CAS PubMed; (c) S. Cenini, E. Gallo, A. Penoni, F. Ragaini and S. Tollari, Chem. Commun., 2000, 2265–2266 RSC; (d) Q. Nguyen, T. Nguyen and T. G. Driver, J. Am. Chem. Soc., 2013, 135, 620–623 CrossRef CAS PubMed; (e) F.-C. Jia, C. Xu, Z.-W. Zhou, Q. Cai, D.-K. Li and A.-X. Wu, Org. Lett., 2015, 17, 2820–2823 CrossRef CAS PubMed.
- (a) J. Shen, D. Yang, Y. Liu, S. Qin, J. Zhang, J. Sun, C. Liu, C. Liu, X. Zhao, C. Chu and R. Liu, Org. Lett., 2014, 16, 350–353 CrossRef CAS PubMed; (b) K. Wang, K. Nguyen, Y. Huang and A. Dömling, J. Comb. Chem., 2009, 11, 920–927 CAS.
Footnotes |
| † Presented in International Conference on “Nascent Development in Chemical Sciences: Opportunities for Academia-Industry Collaboration” at BITS Pilani during October 16–18, 2015 and selected for best poster award sponsored by RSC. |
| ‡ Electronic supplementary information (ESI) available: Copies of 1H NMR, 13C NMR spectra for all the synthesized compounds and tables for single X-ray data of 10. CCDC 1433055. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra03798d |
| This journal is © The Royal Society of Chemistry 2016 |