Morphology-controlled synthesis of SrTiO3/TiO2 heterostructures and their photocatalytic performance for water splitting†
Minh Ngoc Haab,
Feng Zhub,
Zhifu Liu*b,
Lichao Wangb,
Linyan Liub,
Guanzhong Luab and
Zhe Zhao*bc
aKey Laboratory for Advanced Materials, Research Institute of Industrial Catalysis, East China University of Science and Technology, Shanghai 200237, China
bDepartment of Materials Science and Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: liuzf@sit.edu.cn; zhezhao@kth.se; Fax: +86-21-60873439; Tel: +86-21-60877203
cDepartment of Materials Science and Engineering, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
First published on 15th February 2016
Abstract
Different morphologies of SrTiO3/TiO2 heterostructures like nanocubes, nanoparticles, nanospheres, and nanofibers were synthesized via a facile hydrothermal process using TiO2 as both a template and precursor in Sr(OH)2 solution. Their structure, interface and composition can be rationally tailored by simply adjusting the Sr(OH)2/TiO2 (Sr/Ti) mole ratios and the morphology of SrTiO3/TiO2 heterostructures can be controlled easily using TiO2 with different morphologies. A SrTiO3 crystal thin layer was grown on an anatase TiO2 substrate to fabricate a heterostructure interface contact between SrTiO3 and TiO2 and the lattice mismatch had an effect on the electrical transport properties. The SrTiO3/TiO2 heterostructures are beneficial for the fast separation of photogenerated electrons and holes so as to suppress the recombination of photogenerated electrons and holes at the interface of SrTiO3 and TiO2. Besides this, the different morphologies of the SrTiO3/TiO2 heterostructures allowing facile electron transfer, the hierarchical structure promoting mass transfer and allowing more light reflection and absorption, and the large specific surface area providing more reaction sites to facilitate the reactants to the desired oxidation places all together create a synergistic effect to improve the photocatalytic activity of the hierarchical SrTiO3/TiO2 heterostructures. Under the irradiation of UV light, in a water/methanol sacrificial reagent system, the SrTiO3/TiO2 NP heterostructures at a Sr/Ti mole ratio of 40% with the highest BET and smallest crystallite size achieve the highest photocatalytic activity generating 0.731 mmol of H2. The SrTiO3/TiO2 heterostructures exhibit better photocatalytic activity by generating three times more H2 than bare TiO2 and pure SrTiO3.
Introduction
Nowadays, fossil fuels are still the main energy resource for our society. However, the shortage of fossil fuels and the growing environmental concerns due to the emission of large amounts of CO2 during the combustion of fossil fuels have become global problems. How to provide clean, affordable, and reliable energy without causing climate change is a common concern in the world.1 Hydrogen (H2) energy has proved to be a promising clean energy source owing to its intrinsic properties, such as being pollutant-free and having a high energy density, and thus has attracted a huge amount of research efforts all over the world.2 Photocatalytic water splitting with catalyst particles suspended in water has been intensively studied because the process presents a clean and renewable source for hydrogen energy.3 TiO2 was the first material used for an electrochemical water splitting reaction by Fujishima and Honda in 1972 under ultra-violet radiation.4 However, the high recombination of the photo-generated charge carriers leads to the low photocatalytic activity of TiO2-based nanomaterials. In order to overcome this drawback, forming a heterojunction structure by combining TiO2 with another semiconductor is considered to be one of the efficient ways to suppress the recombination of photo-excited electron–hole pairs and to enhance the photocatalytic efficiency.5,6 Particularly, the fabrication of TiO2-based heterostructures such as TiO2/Ag,7 CdS/TiO2,8 TiO2/CeO2,9 Nb2O5/TiO2,10 CdSe/TiO2,11 TiO2/SnO2,12 and TiO2/Bi2WO6,13 has received increasing attention recently due to their promotion of the separation of photogenerated electron–hole pairs and thus their enhancement of the photocatalytic activity of pure TiO2.Besides, SrTiO3 is a well-known cubic-perovskite-type photocatalyst with a band gap of 3.2 eV and possesses superior chemical stability.14,15 Coupling SrTiO3 to TiO2 can be expected to introduce a strong interface electric field by band-edge offset,16–21 which will effectively accelerate the separation of photo-generated carriers and improve the photocatalytic performance of TiO2. SrTiO3 has been synthesized through a variety of methods, including sol–gel methods,22 hydrothermal synthesis,23,24 conventional solid-state reactions,25 inverse micelle micro-emulsion methods,26 molten salt synthesis,27 and nonaqueous routes.28 Recently, compounds with perovskite-type structures have emerged from hydrothermal reactions as an important conformation. Therefore, it would be highly desirable to develop a general approach to fabricate SrTiO3/TiO2 heterostructures with controllable morphology and size. Furthermore, the activity of heterogeneous catalysts is essentially influenced by the crystal phase, size, surface area, crystallinity, and especially the exposed crystal facets.29–32 Their morphology and outer surface also affect their photocatalytic activity.33 In this regard, the precise control of the size and shape of SrTiO3 is critically important for evaluating the shape-dependent photo-reactivity and developing a high-performance photocatalyst. However, in spite of the previous extensive efforts, such a precise control of their size and shape has been fragmented and limited. The studies on the effect of the SrTiO3/TiO2 heterostructure interface thickness and different morphologies for hydrogen generation have not been reported.
Herein, we would like to report the synthesis of different interface thicknesses of SrTiO3/TiO2 nanoparticles by adjusting the Sr/Ti mole ratios, and morphology-controlled SrTiO3/TiO2 nanocube (NC), nanoparticle (NP), nanosphere (NS), and nanofiber (NF) heterostructures, via a facile hydrothermal process using the different morphologies of TiO2 as both templates and precursors in Sr(OH)2 solution. The synthesis process is facile, low cost, and environmentally-friendly. In addition, the effects of the interface thickness and different morphologies on the photocatalytic activity of SrTiO3/TiO2 heterostructures for hydrogen production from water have been investigated. Under the irradiation of ultra-violet (UV) light, in a water/methanol sacrificial reagent system, the SrTiO3/TiO2 heterostructures exhibit much better efficiency on hydrogen production compared with the bare TiO2 and pure SrTiO3.
In a typical experiment, a series of SrTiO3/TiO2 NP heterostructures with different mole ratios, Sr/Ti = 0, 20, 40, 60, 80, and 100%, and different morphology-controlled SrTiO3/TiO2 NC, NP, NS, and NF heterostructures with mole ratios of Sr/Ti = 40%, were prepared by a hydrothermal method at 160 °C for 12 h. The well-prepared SrTiO3/TiO2 heterostructures were investigated for photocatalytic H2 generation in a methanol/water sacrificial reagent system under the irradiation of UV light. Finally, the H2 gas generated from the photocatalytic reaction was analyzed using TCD-type gas chromatography (GC-7900 with a 5A molecular sieve column) (ESI S1†).
Results and discussion
XRD patterns
XRD analysis was conducted to reveal the crystal structures of SrTiO3/TiO2 heterostructures. Fig. 1a shows the XRD patterns of SrTiO3/TiO2 NP samples prepared by a hydrothermal process with different mole ratios of Sr/Ti = 0, 20, 40, 60, 80, and 100%. From the XRD measurements, it can be seen that the XRD data of the SrTiO3/TiO2 NPs match exactly with the standard TiO2 anatase (space group I41/amd, Ref. code 01-089-4921) and cubic perovskite SrTiO3 (space group Pm3m, Ref. code 01-074-1296). The XRD pattern of bare TiO2 NPs (Sr/Ti = 0%) in Fig. 1a shows diffraction peaks at 2θ = 25.39°, 37.99°, 48.09°, 53.95°, 55.11°, and 62.77°, which could be perfectly indexed to the (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1), and (2 0 4) crystal faces of anatase TiO2, respectively. After being hydrothermally treated in Sr(OH)2 solution with Sr/Ti = 20, 40, 60, 80 and 100% at 160° for 12 h, additional diffraction peaks with 2θ = 22.68°, 32.34°, 39.88°, 46.43°, 57.74°, and 77.12° appeared, corresponding to (1 0 0), (1 1 0), (1 1 1), (2 0 0), (2 1 1), (2 2 0), and (3 1 0) crystal planes of cubic perovskite SrTiO3, respectively. It was indicated that part of TiO2 successfully converted into SrTiO3.34 Besides, when the mole ratio of Sr/Ti increases, the diffraction peaks of anatase decline and the diffraction peaks of SrTiO3 appear gradually and become stronger. With the mole ratio of Sr/Ti = 100%, the diffraction peaks of anatase completely disappeared and only pure SrTiO3 diffraction peaks were present. A SrTiO3 crystal thin layer was grown on an anatase TiO2 substrate to fabricate the heterostructure interface contact between SrTiO3 and TiO2. This result solidly confirmed that the SrTiO3/TiO2 heterostructures have formed through the hydrothermal process. Moreover, when the mole ratio of Sr/Ti increased, the SrTiO3 layer was thicker, affecting the electrical transport properties. Through adjusting the Sr/Ti mole ratios and morphology, we can easily control the SrTiO3/TiO2 interface and SrTiO3 nano-layer. | ||
| Fig. 1 (a) XRD patterns of SrTiO3/TiO2 heterostructures prepared with different Sr/Ti mole ratios, (b) XRD patterns of the different morphologies of the SrTiO3/TiO2 heterostructures. | ||
Fig. 1b shows the XRD patterns of the hydrothermally synthesized different morphologies of the SrTiO3/TiO2 NC, NP, NS, and NF heterostructures with mole ratios of Sr/Ti = 40%. It was evident that all of the patterns showed good crystallinity with the diffraction peaks fitting well with the peak positions of standard TiO2 anatase and standard cubic perovskite SrTiO3 structures. From the XRD analysis in Table 1, it can be seen that the smallest crystallite size of the SrTiO3/TiO2 NP heterostructures was 45.04 nm. The results could correspond to the highest photocatalytic performance below and according to previous research35,36 this may be because small crystallites have a larger external surface, which offers a higher number of active sites and enhances the reaction occurring above the active sites compared with large crystallites.
| Catalyst SrTiO3/TiO2 heterostructures | 2-Theta (degree) | d1 1 0 (nm) | Crystallite size (nm) | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) |
|---|---|---|---|---|---|
| Rietveld structure refinement for SrTiO3 | |||||
| NC | 32.397 | 0.276 | 71.652 | 11.783 | 0.040 |
| NP | 32.335 | 0.277 | 45.041 | 23.195 | 0.079 |
| NS | 32.334 | 0.277 | 93.489 | 15.947 | 0.082 |
| NF | 32.342 | 0.277 | 50.811 | 17.560 | 0.060 |
The specific surface area, pore structures, and size distributions of the SrTiO3/TiO2 NC, NP, NS, and NF heterostructure samples were characterized by nitrogen adsorption–desorption isotherms at 77 K (Fig. 2 and Table 2 S1†). It is seen that the SrTiO3/TiO2 heterostructure samples showed a mesoporous structure for all of the different morphologies. The nitrogen adsorption–desorption isotherms can be classified as type III isotherms, typical of mesoporous materials. According to IUPAC classification, the hysteresis loop is type H3. This type of hysteresis is usually found for solids consisting of aggregates or agglomerates of particles forming slit shaped pores, with a non-uniform size and/or shape. The BET specific surface area of the SrTiO3/TiO2 NP heterostructures is the highest at 23.195 m2 g−1, and characteristic of mesoporous materials, with an average Barrett–Joyner–Halenda (BJH) pore diameter of 13.63 nm and a total pore volume of 0.079 cm3 g−1. The high BET surface area and large total pore volume strongly support the fact that the SrTiO3/TiO2 NPs have a mesoporous structure. The enlarged specific surface area would create more reaction sites to facilitate the access of reactants. Considering the abovementioned advantages, it is reasonable to believe that the hierarchical SrTiO3/TiO2 NP heterostructures would be favorable for the improvement of the photocatalytic activity more than SrTiO3/TiO2 NC, NS, and NF heterostructures.
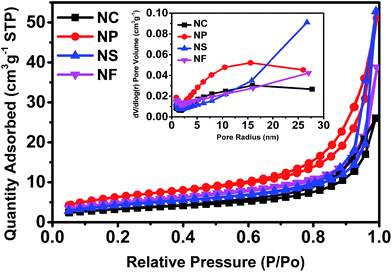 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherm plot and pore size distribution of SrTiO3/TiO2 NC, NP, NS, and NF structures. | ||
SEM images
The morphologies of SrTiO3/TiO2 NC, NP, NS and NF heterostructures were observed using SEM. Fig. 3 shows the SEM images of the TiO2 precursors (left side) and SrTiO3/TiO2 samples (right side) that were obtained at different morphologies. The bare anatase TiO2 NC, NP, and NS structures are highly ordered with sizes of about 650, 295, and 390 nm, respectively. Anatase TiO2 NF structure appears to be a long tube structure with a length of about five micrometres and a diameter of less than 310 nm. From the SEM images, it can be clearly seen that the SrTiO3/TiO2 samples (right side) keep the same morphology compared with the corresponding precursors (left side), and the final samples became thicker and rougher. This indicated that in the hydrothermal reaction process, using TiO2 precursor as a template, the SrTiO3 nanoparticle layer could gradually grow on the TiO2 precursor surface and these samples have homogeneous morphologies. | ||
| Fig. 3 SEM images of (a) TiO2 NC, (b) SrTiO3/TiO2 NC, (c) TiO2 NP, (d) SrTiO3/TiO2 NP, (e) TiO2 NS, (f) SrTiO3/TiO2 NS, (g) TiO2 NF, and (h) SrTiO3/TiO2 NF structures. | ||
From our experimental results, we proposed that the formation of the SrTiO3/TiO2 heterostructures might be governed by a dissolution and precipitation mechanism, followed by the Ostwald ripening process. The evolution process is illustrated in Fig. 4. During the first stage, tiny SrTiO3 nanoparticles were produced when the TiO2 nanostructures were immersed in Sr(OH)2 solution. The formation of SrTiO3 nanoparticles by the TiO2 precursor in alkaline solution is proposed to be due to a dissolution and precipitation mechanism that involves the dissolution of titanium oxide followed by the nucleation of the perovskite SrTiO3 crystal.37–39 The Ti–O bonds on the TiO2 precursor must be broken via a hydrolytic attack to form soluble [Ti(OH)6]2− and then the as-formed [Ti(OH)6]2− reacted with Sr2+ to form SrTiO3 that nucleated onto the surface of the TiO2 nanostructures. As the reaction proceeded, the in situ generated SrTiO3 nanoparticles accumulated on the surface of the TiO2 nanostructures to form a SrTiO3 nanoparticle layer. Meanwhile, the larger nanocubes grew at the cost of the small particles, and the reduction in surface energy is the primary driving force for the crystal growth and morphology evolution, which is due to the difference in solubility between the larger particles and small particles, according to the well-known solid-solution-solid process named the Ostwald ripening process. Finally, as the reaction continued further, the nanoparticles vanished due to diffusion into the growing nanocubes, and larger nanocubes were finally formed on the surface of the TiO2 nanoprecursors.
 | ||
| Fig. 4 Schematic illustration of the formation process of SrTiO3/TiO2 heterostructures from TiO2 precursors via a hydrothermal reaction with Sr(OH)2. | ||
The formation process of the SrTiO3/TiO2 heterostructures from TiO2 precursors via a hydrothermal reaction with Sr(OH)2 is described in eqn (1)–(3).
| TiO2 + 2OH− + 2H2O → [Ti(OH)6]2− | (1) |
| Sr2+ + [Ti(OH)6]2− → SrTiO3 + 3H2O | (2) |
| TiO2 + Sr(OH)2 → SrTiO3 + H2O | (3) |
TEM images
The TEM images of the SrTiO3/TiO2 heterostructures are shown in Fig. 5. It can be observed from Fig. 5a, d, g and k that the small SrTiO3 nanoparticles with an average size of 20–50 nm were well-distributed on the surface of the TiO2 NC, NP, NS, and NF structures. In combination with the XRD, SEM and the EDS analysis below, it can be inferred that the SrTiO3 nanoparticles were well crystallized on the surface of the bare TiO2. | ||
| Fig. 5 TEM images and EDS map images of (a–c) SrTiO3/TiO2 NC, (d–f) SrTiO3/TiO2 NP, (g–i) SrTiO3/TiO2 NS, and (j–l) SrTiO3/TiO2 NF structures. | ||
Moreover, it can be seen in Fig. 5b, e, h and k that there is a clear interface between SrTiO3 and TiO2, which may correspond to the heterostructures of the SrTiO3/TiO2 NCs, NPs, NSs, and NFs. The TEM images in Fig. 5b, e, h and k also show two kinds of clear lattice fringes. The spacing of one of the fringes was 0.35 nm, corresponding to the (1 0 1) plane of the anatase crystal structure of TiO2. The other kind of fringe spacing was 0.27 nm, corresponding to the (1 1 0) lattice spacing of the cubic phase of SrTiO3.
A Bruker-AXS 133 eV XFlash 4010 Detector attached to the SEM was used to measure the element composition and distribution of the SrTiO3/TiO2 heterostructures. From the EDS spectrum and the element mapping images of O (red), Ti (green) and Sr (blue) in Fig. 5c, f, i and l, it can be clearly seen that O, Sr, and Ti dominate the composition of the SrTiO3/TiO2 heterostructures. Those mapping images are solid proofs that O, Sr and Ti are uniformly distributed in the SrTiO3/TiO2 heterostructures. This further consolidates that SrTiO3 and TiO2 are in close contact to each other, which is in favor of the separation of photogenerated electrons and holes to promote the photocatalytic activity improvement.40
UV-vis analysis
The optical properties of the different morphologies of the SrTiO3/TiO2 heterostructure samples were investigated using UV-vis diffuse absorption spectra. Fig. 6a shows the diffuse absorption spectra of the SrTiO3/TiO2 NC, NP, NS, and NF heterostructures. The band gap energy (Eg) of the SrTiO3/TiO2 heterostructure samples was calculated from the absorption data using the Tauc relation. The average band gap was estimated from the intercept of the linear portion of the (εhν)1/2 vs. hν plots on the hν axis as shown in Fig. 6b. The band gap of the SrTiO3/TiO2 heterostructures with a mole ratio of Sr/Ti = 40% is estimated to be 3.09, 3.12, 3.04, and 3.10 eV for NC, NP, NS, and NF heterostructure samples, respectively. Fig. 6b also shows that the band gap of pure anatase TiO2 NPs is 2.95 eV and 3.18 eV for pure SrTiO3 NPs. The band gap of the different morphologies of the SrTiO3/TiO2 heterostructure samples is larger than the band gap of pure anatase TiO2 and smaller than pure SrTiO3. Their photocatalytic properties might exist under the UV light irradiation. The SrTiO3/TiO2 heterostructures were considered an indirect band gap semiconductor.41,42 Interestingly, the SrTiO3 crystal thin layer was grown on an anatase TiO2 substrate to fabricate the heterostructure interface, which changed the lattice mismatch. The Sr-doping into the lattice of TiO2 and the formation of Sr-doped SrTiO3/TiO2 heterostructures or self-doped SrTiO3 may favor creating an impurity energy level, modifying the optical response properties and narrowing the band gap for a wide light absorption.43,44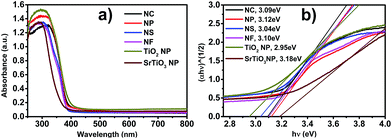 | ||
| Fig. 6 UV-vis spectra (a) and (b) Tauc plots for the determination of optical band gap for SrTiO3/TiO2 NC, NP, NS and NF heterostructures. | ||
Photocatalytic performance
The H2 generation activities of the SrTiO3/TiO2 heterostructures were evaluated under the irradiation of UV light in a water/methanol sacrificial reagent system. Fig. 7a displays the H2 evolution curves of SrTiO3/TiO2 NP heterostructure samples synthesized with different Sr/Ti = 0, 20, 40, 60, 80, and 100% mole ratios under the same conditions. The observed H2 generation activities of the SrTiO3/TiO2 NP heterostructures were in the order of the mole ratios (40% > 60% > 80% > 100% > 0%). Obviously, the SrTiO3/TiO2 NP heterostructures exhibit better photocatalytic H2 generation activity than pure SrTiO3 with Sr/Ti = 100% and pure TiO2 with Sr/Ti = 0%. During the same reaction period, the SrTiO3/TiO2 NP heterostructures with mole ratios of Sr/Ti = 40% showed the highest photocatalytic activity. Three times more H2 was generated than with pure TiO2. The SrTiO3/TiO2 NP heterostructures with Sr/Ti = 100% (pure SrTiO3) produced a larger amount of hydrogen from water than SrTiO3/TiO2 NP heterostructures with Sr/Ti = 0% (pure TiO2). The result may suggest that both the pure SrTiO3 and TiO2 have similar band gaps. The valence band level is also identical in both materials. Since the valence band levels are the same, the water oxidation efficiency will be the same. The only major difference is that the conduction band level of SrTiO3 is slightly more negative than that of TiO2. It means that the thermodynamic driving force for the transfer of photoexcited electrons will be slightly higher in SrTiO3. So, hydrogen production by water reduction may be more efficient on SrTiO3 compared to TiO2. What is more, the synthesized SrTiO3/TiO2 heterostructure NPs with different Sr/Ti mole ratios changed the interface thickness, facilitated electron transfer, and affected the photocatalytic activity, and the structural defects in the particles of the heterostructures are believed to play an essential role in the photocatalytic reaction.Fig. 7b shows the photocatalytic H2 generation of the different morphologies of the SrTiO3/TiO2 NC, NP, NS, and NF heterostructures prepared with mole ratios of Sr/Ti = 40% under the same reaction conditions. The different morphologies of the SrTiO3/TiO2 heterostructures influence the amount of H2 evolution. On the other hand, the different morphologies also give a different crystallite size, surface area, bandgap, and interface promotion effect on the catalytic activity in the following sequence of the SrTiO3/TiO2 heterostructures, NP > NF > NC > NF, as we already observed. The SrTiO3/TiO2 NP heterostructures show the highest amount of H2 generation, 0.731 mmol, under UV irradiation for 8 h, more details in Table 3 (ESI S1†). Moreover, the band gap energy is also correlated to the photocatalytic activity.45 The lower band gap has a positive effect on the photocatalytic activity because a lower source energy is needed for inducing a photocatalytic reaction. It means that less energy is needed to activate the nanoparticles to generate excited electron/hole pairs and then induce photocatalytic reactions. In this study, the band gap values of the SrTiO3/TiO2 NC, NP, NS, and NF heterostructures are smaller than reported for pure SrTiO3 nanoparticles of 3.2 eV.15 In addition, the BET specific surface area of the SrTiO3/TiO2 NP heterostructures is 23.195 m2 g−1, which is the largest and its crystallite size is the smallest at 45.04 nm. It was known that the size of the nanoparticles had significant effects on the photocatalytic properties due to the variation of surface area, the number of active sites, and so on.46 The smaller particle size of the nanoparticles would induce a larger surface area (more active sites) to enhance the photocatalytic activity. The results indicate that the SrTiO3/TiO2 heterostructures with a larger surface area, small crystallite size and band gap energy that changed to smaller values than pure SrTiO3 can efficiently improve the water splitting photocatalytic performance.
Mechanism of photocatalytic water splitting
A schematic diagram of the energy-level configuration and the photogenerated charge-transfer process in the SrTiO3/TiO2 heterostructures is illustrated in Fig. 8 according to previous reports.43,47,48 The SrTiO3/TiO2 heterostructures with different morphologies and hierarchical structure all play positive roles in the separation process of the photogenerated electrons and holes. The enhanced photocatalytic performance of the SrTiO3/TiO2 heterostructures can be attributed to the presence of nano–nano heterojunctions,34 which may accelerate the separation of photogenerated electron–hole pairs in the SrTiO3/TiO2 heterostructures. Under the irradiation of UV light, electrons (e−) in the conduction band of SrTiO3 were excited and fast transferred to that of TiO2 in the SrTiO3/TiO2 heterostructures. The electrons were scavenged by protons (h+), forming H2. Meanwhile, the remaining holes in the valence band of TiO2 were transferred to that of SrTiO3. Because of the higher conduction bond minimum (CBM) and valence bond maximum (VBM) of SrTiO3 than those of TiO2, under the excitation of ultraviolet light, the photogenerated electrons on the CBM of SrTiO3 can be readily injected into the CBM of TiO2, while the photogenerated holes on the VBM of TiO2 can directly transfer to the VBM of SrTiO3. The efficient charge separation can increase the lifetime of the charge carriers and improve the efficiency of the interfacial charge transferred to the adsorbed substrates, resulting in a higher activity of the SrTiO3/TiO2 heterostructure photocatalyst. In addition, the hydrothermal growth of SrTiO3 in situ by employing TiO2 as one of the precursor materials guarantees the close contact of SrTiO3 nanoparticles with TiO2, which can effectively facilitate the charge transport process.47 In this water/methanol sacrificial reagent system, methanol acts as a scavenger to scavenge the holes and was finally mineralized.48 In such a way, the presence of a heterostructure interface meant that the recombination of photogenerated electrons and holes was suppressed effectively, and the photocatalytic activity was greatly enhanced. Furthermore, the hierarchical structure promoting mass transfer and allowing more light reflection and absorption, along with the large specific surface area and small crystallite size providing more reaction sites to facilitate the reactants to the desired oxidation places, all together create a synergistic effect to improve the photocatalytic activity of the hierarchical SrTiO3/TiO2 heterostructures. | ||
| Fig. 8 Schematic of the photoexcited electron transfer and H2 evolution on the SrTiO3/TiO2 NP photocatalyst under UV irradiation (H+ scavenged by CH3OH as a sacrificial reagent). | ||
Conclusions
In this study, we successfully prepared SrTiO3/TiO2 NC, NP, NS, and NF heterostructures via a facile hydrothermal process using TiO2 both as templates and initial reactants under 160 °C for 12 h in Sr(OH)2 solution. Their structure, interface, phase components and surface area can be rationally tailored by simply adjusting the molar ratio of the Sr and Ti precursors, and the morphology of the SrTiO3/TiO2 heterostructures was controlled using TiO2 with different morphologies. The chemical composition of the components and nanostructures of the SrTiO3/TiO2 heterostructures were investigated using XRD, SEM, TEM, EDS, BET and UV-vis characterization techniques. Compared with pure TiO2 and SrTiO3, the SrTiO3/TiO2 heterostructures exhibit better photocatalytic activity in water splitting, generating three times the amount of H2. The particle size, interface and morphology of the SrTiO3 nanostructures were found to dominate the photocatalytic activity of the SrTiO3/TiO2 heterostructures through regulating the adhesion and aggregation of TiO2 as well as the number of active reaction sites. Under the irradiation of UV light, in a water/methanol sacrificial reagent system, the SrTiO3/TiO2 NP heterostructures at a Sr/Ti mole ratio of 40% with the highest BET and smallest crystallite size achieved the highest photocatalytic activity, generating 0.731 mmol of H2 in 8 h. The research reported here proposes a novel method to fabricate SrTiO3/TiO2 heterostructures on limited two-dimensional NCs, NPs, NSs, and NFs in situ, which is beneficial for understanding the growth of SrTiO3 from TiO2 in the initial period, to fabricate a heterostructure interface contact between SrTiO3 and TiO2 causing lattice mismatch which has an effect on the electrical transport properties. These results may further promote the applications of SrTiO3/TiO2 heterostructures in catalysis, environment cleanup, solar cells, sensors, and photonic and optoelectronic devices.Acknowledgements
We gratefully acknowledge financial support by the program for young scientists (YangFan Program, 14YF1410800) at the Science and Technology Commission of Shanghai Municipality, young teachers training scheme of the Shanghai Municipal Education Commission (ZZyy15085, ZZyy15086), the program of introducing talents of the Shanghai Institute of Technology (YJ2014-42) and the special fund to support the development of local colleges of the Ministry of Finance of China.Notes and references
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- M. Ashokkumar, Int. J. Hydrogen Energy, 1998, 23, 427–438 CrossRef CAS.
- Z. Zou, J. Ye and H. Arakawa, Chem. Phys. Lett., 2000, 332, 271 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- Y. B. Chen, L. Z. Wang, G. Q. Lu, X. D. Yao and L. J. Guo, J. Mater. Chem., 2011, 21, 5134–5141 RSC.
- Y. B. Chen and L. J. Guo, J. Mater. Chem., 2012, 22, 7507–7514 RSC.
- Z. Shan, J. Wu, F. Xu, F. Huang and H. Ding, J. Phys. Chem. C, 2008, 112, 15423 CAS.
- X. F. Gao, W. T. Sun, Z. D. Hu, G. Ai, Y. L. Zhang, S. Feng, F. Li and L. M. Peng, J. Phys. Chem. C, 2009, 113, 20481 CAS.
- T. Cao, Y. Li, C. Wang, L. Wei, C. Shao and Y. Liu, Mater. Res. Bull., 2010, 45, 1406 CrossRef CAS.
- H. Cui, K. Dwight, S. Soled and A. Wold, J. Solid State Chem., 1995, 115, 187 CrossRef CAS.
- S. C. Lo, C. F. Lin, C. H. Wu and P. H. Hsieh, J. Hazard. Mater., 2004, 114, 182–190 CrossRef PubMed.
- Z. Y. Liu, D. D. L. Sun, P. Guo and J. O. Leckie, Nano Lett., 2007, 7, 1081 CrossRef CAS PubMed.
- M. Shang, W. Wang, L. Zhang, S. Sun, L. Wang and L. Zhou, J. Phys. Chem. C, 2009, 113, 14727 CAS.
- B. Reihl, J. G. Bednorz, K. A. Müller, Y. Jugnet, G. Landgren and J. F. Morar, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 803–806 CrossRef CAS.
- V. Subramanian, R. K. Roeder and E. E. Wolf, Ind. Eng. Chem. Res., 2006, 45, 2187–2193 CrossRef CAS.
- J. Y. Zhang, Y. H. Wang, J. Jin, J. Zhang, Z. Lin, F. Huang and J. G. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 10317–10324 CAS.
- Y. Yang, K. Lee, Y. Kado and P. Schmuki, Electrochem. Commun., 2012, 17, 56–59 CrossRef CAS.
- Y. T. Zheng, Z. L. Zhang and Y. L. Mao, J. Alloys Compd., 2013, 554, 204–207 CrossRef CAS.
- Z. B. Jiao, T. Chen, J. Y. Xiong, T. Wang, G. X. Lu, J. H. Ye and Y. P. Bi, Sci. Rep., 2013, 3, 2720 Search PubMed.
- S. Park, S. Kim, H. J. Kim, C. W. Lee, H. J. Song, S. W. Seo, H. K. Park, D. W. Kim and K. S. Hong, J. Hazard. Mater., 2014, 275, 10–18 CrossRef CAS PubMed.
- E. Y. Guo and L. W. Yin, J. Mater. Chem. A, 2015, 3, 13390 CAS.
- A. F. Demirörs and A. Imhof, Chem. Mater., 2009, 21, 3002–3007 CrossRef.
- Z. K. Zheng, B. B. Huang, X. Y. Qin, X. Y. Zhang and Y. Dai, J. Colloid Interface Sci., 2011, 358, 68–72 CrossRef CAS PubMed.
- Y. Li, X. P. Gao, G. R. Li, G. L. Pan, T. Y. Yan and H. Y. Zhu, J. Phys. Chem. C, 2009, 113, 4386–4394 CAS.
- S. W. Kim, H. I. Choi, M. H. Lee, J. S. Park, D. J. Kim, D. Do, M. H. Kim, T. K. Song and W. J. Kim, Ceram. Int., 2013, 39, 487–490 CrossRef.
- Y. Wang, H. Xu, X. Wang, X. Zhang, H. Jia, L. Zhang and J. Qiu, J. Phys. Chem. B, 2006, 110, 13835–13840 CrossRef CAS PubMed.
- Y. B. Mao, S. Banerjee and S. S. Wong, J. Am. Chem. Soc., 2003, 125, 15718–15719 CrossRef CAS PubMed.
- M. Niederberger, G. Garnweitner, N. Pinna and M. Antonietti, J. Am. Chem. Soc., 2004, 126, 9120–9126 CrossRef CAS PubMed.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078–4083 CrossRef CAS PubMed.
- J. A. Enterkin, K. R. Poeppelmeier and L. D. Marks, Nano Lett., 2011, 11, 993–997 CrossRef CAS PubMed.
- A. Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- J. Zhang, Y. P. Zhang, Y. K. Lei and C. X. Pan, Catal. Sci. Technol., 2011, 1, 273–278 CAS.
- T. Kimijima, K. Kanie, M. Nakaya and A. Muramatsu, Appl. Catal., B, 2014, 144, 462–467 CrossRef CAS.
- T. P. Cao, Y. J. Li, C. H. Wang, C. L. Shao and Y. C. Liu, Langmuir, 2011, 27, 2946–2952 CrossRef CAS PubMed.
- S. B. Pu and T. Inui, Zeolites, 1996, 17, 334–339 CrossRef CAS.
- D. S. Kim, S. J. Han and S. Y. Kwak, J. Colloid Interface Sci., 2007, 316, 85–91 CrossRef CAS PubMed.
- K. Y. Chen and Y. W. Chen, Powder Technol., 2004, 141, 69–74 CrossRef CAS.
- Y. Xin, J. Jiang, K. Huo, T. Hu and P. K. Chu, ACS Nano, 2009, 3, 3228–3234 CrossRef CAS PubMed.
- C. W. Kim, S. P. Suh, M. J. Choi, Y. S. Kang and Y. S. Kang, J. Mater. Chem. A, 2013, 1, 11820–11827 CAS.
- J. W. Ng, S. P. Xu, X. W. Zhang, H. Y. Yang and D. D. Sun, Adv. Funct. Mater., 2010, 20, 4287–4294 CrossRef CAS.
- Y. Yamada and Y. Kanemitsu, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 121103R CrossRef.
- E. Guo and L. W. Yin, J. Mater. Chem. A, 2015, 3, 13390–13401 CAS.
- R. Tang and L. W. Yin, J. Mater. Chem. A, 2015, 3, 17417 CAS.
- K. Xie, N. Umezawa, N. Zhang, P. Reunchan, Y. J. Zhang and J. H. Ye, Energy Environ. Sci., 2011, 4, 4211–4219 CAS.
- L. Manna, E. C. Scher and A. P. Alivisatos, J. Cluster Sci., 2002, 13, 521–532 CrossRef CAS.
- W. C. Lin, W. D. Yang and S. Y. Jheng, J. Taiwan Inst. Chem. Eng., 2012, 43, 269–274 CrossRef CAS.
- H. W. Bai, J. Juay, Z. Y. Liu, X. X. Song, S. S. Lee and D. D. Sun, Appl. Catal., B, 2012, 125, 367–374 CrossRef CAS.
- S. Xu, J. Ng, A. J. Du, J. Liu and D. D. Sun, Int. J. Hydrogen Energy, 2011, 36, 6538–6545 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03472a |
| This journal is © The Royal Society of Chemistry 2016 |

