Tuning the band-gap of h-boron nitride nanoplatelets by covalent grafting of imidazolium ionic liquids†
Abstract
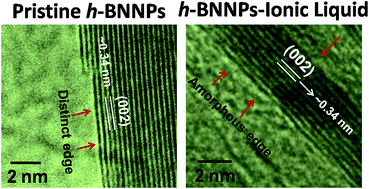
Imidazolium ionic liquids with three different anions, viz. bis(salicylato)borate (BScB), oleate (OL) and hexafluorophosphate (PF6), are covalently grafted on h-boron nitride nanoplatelets (h-BNNPs) to probe the shifts in the band gap energy. The grafting of ionic liquids on h-BNNPs was confirmed by FTIR, XPS, TGA and EDX analyses, whereas XRD and HRTEM results suggested that the crystalline and layering structure of h-BNNPs remained intact after covalent grafting of ionic liquids. The characteristic band gap energy of h-BNNPs (5.9 eV) was reduced to 5.78, 5.25 and 5.00 eV in h-BNNPs-OL, h-BNNPs-PF6 and h-BNNPs-BScB, respectively. The band gap of h-BNNPs-ILs is controlled by charge transfer between the ionic liquids and h-BNNPs, and exhibited strong correlation with the chemical structure of the associated anion in h-BNNPs-ILs.

 Please wait while we load your content...
Please wait while we load your content...