Ultrahigh humidity sensitivity of NaCl-added 3D mesoporous silica KIT-6 and its sensing mechanism†
Xiaowei He,
Wangchang Geng*,
Baoliang Zhang,
Lemin Jia,
Libing Duan and
Qiuyu Zhang*
School of Science, Key Laboratory of Space Applied Physics and Chemistry of Ministry of Education, Northwestern Polytechnical University, Xi'an 710072, People's Republic of China. E-mail: w.geng@nwpu.edu.cn; qyzhang@nwpu.edu.cn
First published on 11th April 2016
Abstract
Mesoporous silica KIT-6 was synthesized by a hydrothermal method and NaCl-added KIT-6 was prepared by a facile grind method. Compared with pure KIT-6, NaCl-added KIT-6 showed greatly improved humidity sensitivity. The impedance changed by more than five orders of magnitude over the whole humidity range (11–95% RH). The response time was about 47 s. Complex impedance spectra were also provided and discussed in detail to explore the humidity sensing mechanism of NaCl-added KIT-6.
Introduction
In recent years, the requirements for humidity measurement have become more and more rigorous in many areas, such as weather forecasting, food storage, the automobile industry, textile technology and so on.1–4 In order to meet the different requirements in different application areas, lots of scientific and technological methods are used to exploit novel humidity sensing materials with high sensitivity, good linearity, fast response, rapid recovery, small hysteresis, long-term stability, and a wide concentration sensing range.5–9 Currently, humidity-sensing material mainly included four categories: organic polymer, ceramic, electrolyte, and porous oxide.10–17 Generally, polymer materials have better flexibility, but their mechanical properties, chemical and thermal stability are poor. In addition, polymer materials are liable to swell and come off from the substrate.18 Although ceramic materials own fairly good chemical and thermal stability, they need longer respond time. For example, a Cr2O3–WO3 composite, reported by Pokhrel et al.,19 shows the response and recovery time as 25 and 7 min, respectively. Despite the sensitivity of electrolyte materials is high, but their measuring range is narrow and have a relatively short lifespan.20–23 Thus, to explore a kind of humidity sensing material which meet most of the requirements for an excellent sensor is still a challenge. Porous oxides, especially mesoporous SiO2, have been widely used as a kind of promising humidity sensing material in recent years24–27 due to their large specific surface area, highly ordered and controllable pore structure and the easily modified wall, which not only provides more active sites for the adsorption of water vapor molecules, but also produces additional diffusion path. Compared with non-mesoporous SiO2, mesoporous SiO2 shows better humidity sensitivity, but the downside is that the linearity of response performance over the entire relative humidity range is defective. In order to improve the linearity of mesoporous SiO2, adding alkali ions was found to be an effective method.23,28 In our previous work,29 humidity sensing properties of Li-doped mesoporous SiO2 was investigated and it was found that humidity sensitivity could be greatly improved by doping LiCl. However, the weakness was that the preparation of Li-doped mesoporous silica needs a heat-treatment under higher temperature (above 500 °C), which would result in high power consumption and the mesoporous structure of silica could be destroyed under this high temperature.25 Therefore, how to overcome this problem is very important to the development of new humidity sensing materials. Recently, we found that using NaCl as the additive to mesoporous silica SBA-15 with 2D hexagonal pore structure could get high humidity sensitivity and meanwhile didn't need the heat treatment.30In this paper, NaCl was used as an additive to mesoporous silica KIT-6 with 3D cubic pore structure. The effect of adding amount of NaCl on humidity sensing property was studied. Besides the merit that this material need low power consumption during its preparation process, study results showed that NaCl-added 3D KIT-6 possesses higher sensitivity than NaCl-added 2D SBA-15 we have reported.30 The impedance of NaCl-added 3D KIT-6 changed more than five orders of magnitude over the whole humidity range (11–95% RH). This work provides a simple and effective method to get a kind of promising humidity sensing material with ultrahigh sensitivity and meanwhile need low power consumption during the preparation process. Sensing mechanism under different relative humidity was discussed in detail based on the complex impedance plot and equivalent circuits. This sensing mechanism can be extended to explain the sensing performance of other mesoporous materials-based humidity sensors.
Experimental
Preparation of mesoporous silica KIT-6
Mesoporous silica KIT-6 was synthesized according to the method reported by Karen Wilson et al.,31 using the triblock copolymer surfactant EO20PO70EO20 (P123) as a template. The detailed procedure was as follows: 1.0 g P123 was dissolved in a mixture solution of 34.1 g deionized water and 1.9 g hydrochloric acid (36%) under stirring. Subsequently, 1.0 g butanol was added under stirring at 35 °C. After 1 h, 2.15 g tetraethyl orthosilicate TEOS solution was added dropwise under stirring at 35 °C. Then, the resultant mixture was transferred to the Teflon-lined autoclave and treated hydrothermally at 100 °C for 24 h. The product was collected by suction filtration and washed with ethyl alcohol, then dried at 100 °C for 12 h. Finally, the pure mesoporous KIT-6 was obtained by heating the as-prepared sample in air at 500 °C for 5 h with a heating rate of 1 °C min−1 to remove the surfactant template.Preparation of Na-KIT-6
The preparation method of Na-KIT-6 was as follows: NaCl and pure mesoporous KIT-6 with different weight ratio was mixed and ground for 0.5 h. The final product was recorded as Na-KIT-6(X), where the X represents different weight ratios of NaCl to KIT-6. In our work, the values of X are 0.05, 0.1, 0.14, 0.2 and 0.5 for five Na-KIT-6(X) samples, respectively.Characterization
The nitrogen adsorption–desorption isotherms and pore size distribution charts of samples were obtained on TriStarII3020 instrument (MICRO Corporation, America) at −196.15 °C. Infrared spectra were measured on a Bruker Tensor 27 FT-IR spectrophotometer (Bruker Corporation, Germany) using KBr as a carrier. Scanning electron micrographs were observed by VEGA3-LMH (TESCAN Corporation, Czech) after spraying by a JOEL JFC-1600 Ion sputtering instrument. The XRD patterns were measured on an X'Pert Pro MPD X-ray diffraction instrument (PANalytical Co., Holland) at 40 kV and 35 mA. Humidity sensing characterization was performed on a TH2817A model LCR analyzer (Changzhou, China). The powder sample was first mixed with a small amount of ethanol to form a paste. Subsequently, the paste was dip-coated on a ceramic substrate with three pairs of Au interdigitated electrodes (Fig. S1†). Finally, the simple humidity sensor with thickness of about 100 μm (Fig. S2†) was prepared after the coated electrode was dried. The sensors were placed in different controlled humidity environments under room temperature (25 °C ± 2 °C), which were implemented by super-saturated aqueous solutions of different salts of LiCl, KAc, MgCl2, Mg(NO3)2, NaCl, KCl and KNO3, and the corresponding relative humidity (RH) values were 11%, 22%, 43%, 54%, 75%, 85% and 95% RH, respectively. RH value was calibrated by a humidity meter (CEM, DT-625, Shenzhen everbest machinery industry Co. Ltd., China), and the measurement accuracy was ±2% RH and ±2.5% RH in the range of 20–80% RH and other RH range, respectively. The schematic map of experimental apparatus is shown in Fig. 1.Result and discussion
Structure and morphology
The SEM images of KIT-6 and Na-KIT-6(0.2) indicate that both of them have irregular particle morphology (Fig. S3†). Fig. 2a shows the wide angle XRD patterns of pure KIT-6 and Na-KIT-6(0.2). As we can see, a broad peak centered at 22.0° of 2θ is observed for pure KIT-6 (Fig. 2a(II)), corresponding to the amorphous silica of KIT-6. After adding NaCl into KIT-6, the broad peak still be present (Fig. 2a(I)), two intensive peaks are also visible which correspond to the (200) and (220) diffraction of NaCl crystallite.Fig. 2b shows the nitrogen adsorption–desorption isotherms of KIT-6 and Na-KIT-6(0.2). BET surface area, pore volume, and pore size are also characterized. According to the IUPAC Classification,32 these isotherms are apparently of type IV that emerged a sharp capillary condensation step within the range of 0.6–0.8 P/P0 and with H1-type hysteresis loop. Hence, KIT-6 and Na-KIT-6(0.2) are both typically mesoporous structure and with a narrow pore-size distribution. The average pore size and pore volume of Na-KIT-6(0.2) were 6.65 nm and 0.62 cm3 g−1, respectively, which are reduced compared with the corresponding data for pure KIT-6 (pore size: 7.04 nm, pore volume: 1.33 cm3 g−1), and the BET surface area also decreased from 790.6 m2 g−1 for KIT-6 to 386.8 m2 g−1 for Na-KIT-6(0.2). These results confirmed the successfully adding of NaCl into KIT-6.
IR spectra of pure KIT-6 and Na-KIT-6(0.2) are shown in Fig. 2c. Both KIT-6 and Na-KIT-6(0.2) have the absorption peaks at 1082 cm−1 and 819 cm−1, which is ascribed to the asymmetric and symmetric stretching vibration modes of Si–O–Si bond, respectively. For Na-KIT-6(0.2), the characteristic peaks of Si–OH can be observed at 1632 cm−1 and 963 cm−1. Nevertheless, the peak of 963 cm−1 attributed to a stretching mode of free surface silanol is very weak in the curve of pure KIT-6. According to the literature,29,33–35 this is because that the number of free surface hydroxyls on the pore walls of KIT-6 decreased after the sample was calcinated at 550 °C for 6 h. After doping with NaCl, the water absorbency of mesoporous silica was improved, more water molecules were adsorbed on the pore walls of KIT-6 and interacted with Si–O–Si bond to form the surface hydroxyls again. Therefore, the peak intensity at 963 cm−1 for sample Na-KIT-6 is stronger. In addition, shoulder peaks at 1651 cm−1 and 1606 cm−1 are also visible for sample Na-KIT-6 but invisible for sample KIT-6, and the peak at 819 cm−1 also moved to short wavenumber direction for sample Na-KIT-6, this maybe resulted from the interaction between NaCl and mesoporous silica.
Humidity sensing property
Fig. 3a shows the dependence of impedance on relative humidity (RH) for pure KIT-6 and Na-KIT-6(X) (X = 0.01, 0.1, 0.14, 0.2, 0.5) samples. As can be seen, the impedance of all samples decreased with the increasing RH under the same RH, the impedance of the sensing materials attends to decrease with the increasing NaCl content. It should be noted that the impedance of pure KIT-6 under low RH (less than 43% RH) can't be detected because its impedance beyond the measure scale of our instrument (maximum 100 MΩ). For samples of Na-KIT-6(X), the impedance changed more than four orders of magnitude when RH changes from 11% to 95% RH. Especially, the impedance of sample of Na-KIT-6(0.2) changed more than five orders of magnitude, showing the highest sensitivity. In addition, we also compared the orders of magnitude of impedance change for different mesoporous silica-based humidity sensing materials. As shown in Table 1, the impedance change for most materials reported in literatures are less than five orders of magnitude, displaying lower humidity sensitivity than Na-KIT-6 material reported here. Although the sensing materials of potassium-doped SBA-15 displayed high sensitivity, where impedance changed five orders of magnitude,25,36 it consumed more power energy during the preparation process of the senor because they need post heat-treatment under high temperature (550 °C) as shown in Table 1. By contrast, Na-KIT-6 material reported in present work displays high humidity sensitivity (impedance changed five orders of magnitude), meanwhile, it doesn't need the post heat-treatment which are beneficial to save power energy.| Sensing material | Post heat treatment | Sensitivity orders of magnitude | RH range | Literature |
|---|---|---|---|---|
| Li-SBA-15 | Yes (550 °C) | Three | 11–95% RH | 29 |
| KCl-MCM-41 | Yes (100 °C) | Three–four | 11–95% RH | 37 |
| K2CO3-SBA-15 | Yes (550 °C) | Five | 11–95% RH | 36 |
| KCl-SBA-15 | Yes (550 °C) | Five | 11–95% RH | 25 |
| Li-MCM-41 | Yes (—) | Two–three | 11–95% RH | 23 |
| MgO-SBA-15 | No | Three–four | 11–95% RH | 38 |
| ZnO-SBA-15 | No | Three–four | 11–95% RH | 39 |
| Fe-SBA-15 | No | Four | 11–95% RH | 26 |
| Li-SBA-16 | No | Four–five | 11–95% RH | 28 |
| NaCl-KIT-6 | No | Five | 11–95% RH | This work |
Therefore, we can say that the Na-KIT-6 material can be regarded as a more promising humidity sensing material than other similar materials reported in literature. Fig. 3b shows the relationship between impedance and RH at different frequencies, as can be seen, the impedance value of Na-KIT-6(0.2) decreased with the increasing frequency under low RH. However, under higher RH, the dependence of impedance on frequency is not obvious. As we can see, the curve displayed the best linearity under 100 Hz. So the frequency of 100 Hz is determined as the operation frequency for other humidity sensing properties of Na-KIT-6(0.2).
As we all know, response time is an extremely important feature in assessing humidity sensors.40,41 In our work, the response and recovery behavior is researched by moving the sensor from the environment of 11% RH into 95% RH repeatedly. Fig. 3c shows the response and recovery curve of sample Na-KIT-6(0.2). The response time or recovery time is defined as the time taken by a sensor to achieve 90% value of the total impedance change.42 The response time obtained from Fig. 3c for Na-KIT-6(0.2) is about 47 s when the RH increase from 11% to 95% RH, and the recovery time Na-KIT-6(0.2) is about 150 s when the RH decrease from 95% to 11% RH, the longer recovery time maybe resulted from that it's difficult to desorption for these water molecules adsorbed by hydrogen bonding on the surface of pore walls where active hydroxyl groups are existed. Cyclic stability between 95% RH and 11% RH of sample Na-KIT-6(0.2) was also characterized as shown in Fig. 3d. Namely, more than 100 cycles were repeated by measuring the response and recovery time between 95% RH and 11% RH of the Na-KIT-6(0.2) sample. As we can see from Fig. 3d, the response and recovery behavior of this sensor material kept being stable even after repeating more than 100 cycles, indicating that this material has good stability. In addition, the long term stability of Na-KIT-6(0.2) at 100% RH was also characterized, the sensor was kept in 100% RH atmosphere, and the impedance was recorded every five days. As shown in Fig. S4.† with the increase of days exposed to 100% RH environment, the impedance of sensor raised slightly, but with the further growth of days, the impedance didn't change significantly. This result demonstrates that the sensor under 100% RH environment is relatively stable within 25 days.
Afterwards, in order to study the stability of NaCl in the sensor materials, we measured the content of Na element twice by Energy Dispersive Spectrometer (Zeiss Ultra 55, Germany). And the results (Table 1S†) show that the average content of Na element has no obvious change after the sensor has been stored for twenty days.
Humidity sensing mechanism
In order to explore the sensing mechanism of sample Na-KIT-6(0.2) under different relative humidity (RH), complex impedance plots and the corresponding equivalent circuits (Fig. 4) are provided to interpret the conductivity and polarization phenomenon occurred in the sensing materials under the existence of water molecules. The values of corresponding elements such as resistor and capacitor in every equivalent circuit were calculated through the nonlinear curve fit method in original software. The detailed values are shown in Table 2. At low RH, a small amount of water molecules are adsorbed onto the hydrophilic silicon hydroxyls through double hydrogen bonding (as shown in Fig. 5a), which restricts the free movement of water molecules. And at this moment, NaCl exists in crystal form and has no contribution to the conductance of the sensing material. Protons transfer by hopping from site to site between the adjacent hydroxyls. Therefore, under low RH (22% RH), the sensing material exhibits large impedance. The complex impedance plot is a semicircle (Fig. 4a) which is typical of the relaxation mechanism expressed by a resistor and a capacitor connected in parallel (inset in Fig. 4a). When RH increases into 43% RH, more water molecules are physically adsorbed through single hydrogen bonding and single or multi water layers are formed (Fig. 5b). Water can be ionized under the electrostatic field to generate lots of hydronium ions (H3O+) which can transfer through the Groutthuss chain reaction (H2O + H3O+ → H3O+ + H2O).43 Therefore, the resistance of sensing film decreased sharply as proved by the detailed values shown in Table 2. Under this RH, water also can penetrate into the interface between sensing film and substrate where ionization phenomenon of water and the diffusion of charge carriers also occurred. So, the resistance and capacitance step from both the sensing film and the sensing film/substrate interface contributed to the impedance of the humidity sensor. This can be confirmed by the complex impedance plot under this RH shown in Fig. 4b. As we can see, the radius of the semicircle under 43% RH decreased compared with that under 22% RH, indicating that the resistance of the sensing film decreased, which was consistent with the values shown in Table 2. Especially, a line can be observed on the right side of the semicircle, the line may step from the sensing film/substrate interface conductivity. As a result, the humidity sensing mechanism under this RH can be modelled by the equivalent circuit shown in inset of Fig. 4b. When the RH increased further to high region (54% RH, 75% RH, 85% RH, and 95% RH), more and more water molecules were gradually adsorbed and bulk liquid water may exist. At this moment, NaCl were dissolved into the bulk liquid water to form an electrolyte solution where dissociated free Na+ and Cl− also contributed to the conductivity of the humidity sensor (Fig. 5c). Therefore, impedance of the humidity sensor came from three parts under the high RH region: the first is the sensing film impedance, the second is the interface impedance, and the third part came from the electrolyte solution. The corresponding sensing mechanism can be represented by the equivalent circuit shown in inset of Fig. 4c and d. From Fig. 4, we also can see that the semicircle gradually trends to be weakened and a straight line curve emerged gradually at the rear of semicircle with the increasing RH, and when the RH reaches to a high value region, the semicircular part of the complex impedance plot gradually disappeared and was replaced by the linear part. Different shape of complex impedance plot under various RH reflected different sensing mechanism which can be represented by different equivalent circuit as shown in inset of Fig. 4. According to these equivalent circuits, the impedance of the sensor under different RH can be expressed by one common equation as follows:where Rf and Cf represent the resistance and capacitance of the intrinsic sensing film, respectively, Ri and Ci the interface resistance and capacitance between sensing film and electrode, Rs and Cs the resistance and capacitance step from the electrolyte solution generated from the dissolving of NaCl in resistance values (Rf, Ri, and Rs) decreased with the increasing RH and all the capacitance values (Cf, Ci, and Cs) increased with RH. The increase of capacitance resulted from the adsorbed liquid water. As can be seen from Table 2, all the increasing adsorbed water molecules which greatly increased the dielectric constant of the sensing material. In addition, from the above equation, we can see that when the RH increases, the increasing capacitance and the decreasing resistance will result in the decrease of impedance, this is consistent with the result shown in Fig. 3a. In addition, in order to confirm the state of NaCl in KIT-6 under different RH as we described above or as shown in Fig. 5, we give the XRD patterns of Na-KIT-6(0.2) under low (Fig. 6a), middle (Fig. 6b) and high (Fig. 6c) RH condition, respectively.
| RH | RH% | 22% | 43% | 54% | 75% | 85% | 95% |
| Value of element | Rf (Ω) | 2.00 × 107 | 4.75 × 105 | 7.87 × 104 | 1.57 × 104 | 2.45 × 103 | 5.10 × 102 |
| Cf (F) | 1.53 × 10−11 | 1.55 × 10−11 | 1.72 × 10−11 | 5.53 × 10−11 | 2.78 × 10−10 | 1.69 × 10−9 | |
| Ri (Ω) | 9.25 × 104 | 3.03 × 104 | 1.49 × 104 | 6.53 × 103 | 3.25 × 103 | ||
| Ci (F) | 6.84 × 10−9 | 8.08 × 10−8 | 1.89 × 10−7 | 7.40 × 10−7 | 1.39 × 10−6 | ||
| Rs (Ω) | 1.26 × 104 | 4.75 × 103 | 738.40 | 248.90 | |||
| Cs (F) | 5.72 × 10−9 | 1.51 × 10−8 | 1.15 × 10−7 | 4.79 × 10−7 | |||
| R-square | 0.9979 | 0.9991 | 0.9961 | 0.9981 | 0.9975 | 0.9989 |
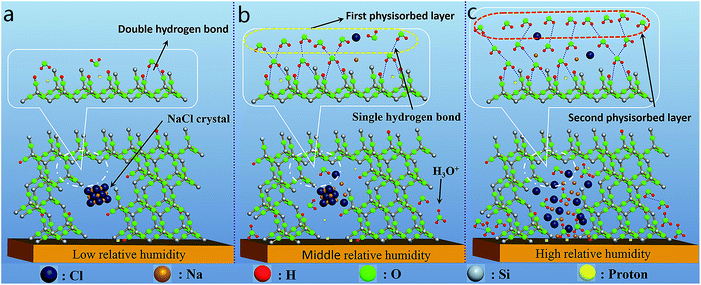 | ||
| Fig. 5 The schematic map for humidity sensing mechanism under (a) low, (b) middle and (c) high RH condition, respectively. | ||
 | ||
| Fig. 6 XRD patterns of Na-KIT-6(0.2) under (a) low, (b) middle and (c) high RH condition. Inset in the magnitude patterns, curves of a′, b′, c′ corresponding to low, middle and high RH, respectively. | ||
As we can see, the typical diffraction peaks (200) and (220) of NaCl are obviously visible when the RH is low (Fig. 6a), indicating NaCl is existed as crystallite under low RH. This is consistent with the schematic map shown in Fig. 5a. Under high RH, as can be seen from Fig. 6c, all the diffraction peaks belonging to NaCl are disappeared, this may be explained by that the NaCl crystallites are dissolved into bulk water molecules under high RH, being agree with what we discussed in sensing mechanism. Under the middle RH, as can be seen from Fig. 6b, all the diffraction peaks of NaCl are visible, indicating the existence of NaCl crystallite. This is also consistent with our analyzation, where we think that only part of NaCl crystallites have been dissolved in water under middle RH (Fig. 5b). Interestingly, it is found that the broad peak belonging to the amorphous silica shifts toward to the high angle side with the increasing RH (from 22.0° of 2θ under low RH to 23.9° under high RH), as shown in inset of Fig. 6, indicating that there is interaction between water molecules and silica, the similar phenomenon was also found in our previous work16 where polypyrrole was used as humidity sensing material. The result from Fig. 6 prove that the state of NaCl in KIT-6 under different RH we described in Fig. 5 are appropriate.
Conclusion
NaCl-added mesoporous silica KIT-6 was prepared and studied as a humidity sensing material. Study results shown that NaCl-added KIT-6 material displayed ultrahigh sensitivity under the proper adding content of NaCl, and it also possessed relatively short response time. The analysis of humidity sensing mechanism indicated that under low RH, intrinsic impedance of the sensing material dominated the conductance of the sensor, under middle RH, both the impedance step from sensing film and the sensing film/electrode interface contributed to the conductance of the sensor, and under high RH, beside the two kinds of impedance under middle RH, the impedance came from the electrolyte solution also contributed to the conductance behavior of the sensor. The sensing mechanism is expecting to be suitable to explain the sensing behavior of other porous material-based humidity sensor.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant No. 21201140), Ph.D. Programs Foundation of Ministry of Education of China (Grant No. 20126102120061), The Fundamental Research Funds for the Central Universities of NPU (Grant No. 3102014JCQ01088) and the Innovative Training Project for Undergraduate Students of NPU (Grant No. 201510699235).Notes and references
- D. Bridgeman, J. Corral, A. Quach, X. Xian and E. Forzani, Colorimetric humidity sensor based on liquid composite materials for the monitoring of food and pharmaceuticals, Langmuir, 2014, 30, 10785–10791 CrossRef CAS PubMed.
- H.-W. Yu, H. K. Kim, T. Kim, K. M. Bae, S. M. Seo, J.-M. Kim, T. J. Kang and Y. H. Kim, Self-powered humidity sensor based on graphene oxide composite film intercalated by poly(sodium 4-styrenesulfonate), ACS Appl. Mater. Interfaces, 2014, 6, 8320–8326 CAS.
- W. Xuan, X. He, J. Chen, W. Wang, X. Wang, Y. Xu, Z. Xu, Y. Q. Fu and J. Luo, High sensitivity flexible lamb-wave humidity sensors with a graphene oxide sensing layer, Nanoscale, 2015, 7, 7430–7436 RSC.
- L. Almar, A. Tarancón, T. Andreu, M. Torrell, Y. Hu, G. Dezanneau and A. Morata, Mesoporous ceramic oxides as humidity sensors: a case study for gadolinium-doped ceria, Sens. Actuators, B, 2015, 216, 41–48 CrossRef CAS.
- J. J. Vijaya, L. J. Kennedy, G. Sekaran and K. Nagaraja, Synthesis, characterization and humidity sensing properties of Cu–Sr–Al mixed metal oxide composites, Mater. Res. Bull., 2008, 43, 473–482 CrossRef CAS.
- P.-G. Su and L.-N. Huang, Humidity sensors based on TiO2 nanoparticles/polypyrrole composite thin films, Sens. Actuators, B, 2007, 123, 501–507 CrossRef CAS.
- Y. D. Park, B. Kang, H. S. Lim, K. Cho, M. S. Kang and J. H. Cho, Polyelectrolyte interlayer for ultra-sensitive organic transistor humidity sensors, ACS Appl. Mater. Interfaces, 2013, 5, 8591–8596 CAS.
- Z. Li, H. Zhang, W. Zheng, W. Wang, H. Huang, C. Wang, A. G. MacDiarmid and Y. Wei, Highly sensitive and stable humidity nanosensors based on LiCl doped TiO2 electrospun nanofibers, J. Am. Chem. Soc., 2008, 130, 5036–5037 CrossRef CAS PubMed.
- S. Wang, Z. Chen, A. Umar, Y. Wang, T. Tian, Y. Shang, Y. Fan, Q. Qi and D. Xu, Supramolecularly modified graphene for ultrafast responsive and highly stable humidity sensor, J. Phys. Chem. C, 2015, 119, 28640–28647 CAS.
- R. I. Molla and J. S. Michael, Poly(N-isopropylacrylamide) microgel-based thin film actuators for humidity sensing, RSC Adv., 2014, 4, 31937–31940 RSC.
- U. Mogera, A. A. Sagade, S. J. George and G. U. Kulkarni, Ultrafast response humidity sensor using supramolecular nanofiber and its application in monitoring breath humidity and flow, Sci. Rep., 2014, 4, 4103 CrossRef PubMed.
- H. Chi, Y. J. Liu, F. Wang and C. He, Highly sensitive and fast response colorimetric humidity sensors based on graphene oxides film, ACS Appl. Mater. Interfaces, 2015, 7, 19882–19886 CAS.
- R. K. Pandey, M. D. Hossain, S. Moriyama and M. Higuchi, Real-time humidity-sensing properties of ionically conductive Ni(II)-based metallo-supramolecular polymers, J. Mater. Chem. A, 2014, 2, 7754–7758 CAS.
- N.-B. Cho, T.-H. Lim, Y.-M. Jeon and M.-S. Gong, Inkjet printing of polymeric resistance humidity sensor using UV-curable electrolyte inks, Macromol. Res., 2008, 16, 149–154 CrossRef CAS.
- S. Taccola, F. Greco, A. Zucca, C. Innocenti, C. de Julián Fernández, G. Campo, C. Sangregorio, B. Mazzolai and V. Mattoli, Characterization of free-standing PEDOT: PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors, ACS Appl. Mater. Interfaces, 2013, 5, 6324–6332 CAS.
- W. Geng, N. Li, X. Li, R. Wang, J. Tu and T. Zhang, Effect of polymerization time on the humidity sensing properties of polypyrrole, Sens. Actuators, B, 2007, 125, 114–119 CrossRef CAS.
- D. M. Li, J. J. Zhang, L. Shen, W. Dong, C. H. Feng, C. X. Liu and S. P. Ruan, Humidity sensing properties of SrTiO3 nanospheres with high sensitivity and rapid response, RSC Adv., 2015, 5, 22879–22883 RSC.
- C.-W. Lee, H.-S. Park, J.-G. Kim, B.-K. Choi, S.-W. Joo and M.-S. Gong, Polymeric humidity sensor using organic/inorganic hybrid polyelectrolytes, Sens. Actuators, B, 2005, 109, 315–322 CrossRef CAS.
- S. Pokhrel and K. Nagaraja, Electrical and humidity sensing properties of Chromium(III) oxide–tungsten(VI) oxide composites, Sens. Actuators, B, 2003, 92, 144–150 CrossRef CAS.
- C.-Y. Lee and G.-B. Lee, Humidity sensors: a review, Sens. Lett., 2005, 3, 1–15 CrossRef CAS.
- Z. Chen and C. Lu, Humidity sensors: a review of materials and mechanisms, Sens. Lett., 2005, 3, 274–295 CrossRef CAS.
- T. Zhang, Y. He, R. Wang, W. Geng, L. Wang, L. Niu and X. Li, Analysis of dc and ac properties of humidity sensor based on polypyrrole materials, Sens. Actuators, B, 2008, 131, 687–691 CrossRef CAS.
- L. Wang, D. Li, R. Wang, Y. He, Q. Qi, Y. Wang and T. Zhang, Study on humidity sensing property based on Li-doped mesoporous silica MCM-41, Sens. Actuators, B, 2008, 133, 622–627 CrossRef CAS.
- V. K. Tomer, S. Duhan, P. V. Adhyapak and I. S. Mulla, Mn-loaded mesoporous silica nanocomposite: a highly efficient humidity sensor, J. Am. Ceram. Soc., 2015, 98, 741–747 CrossRef CAS.
- W. Zhang, R. Wang, Q. Zhang and J. Li, Humidity sensitive properties of K-doped mesoporous silica SBA-15, J. Phys. Chem. Solids, 2012, 73, 517–522 CrossRef CAS.
- Q. Qi, T. Zhang, X. Zheng and L. Wan, Preparation and humidity sensing properties of Fe-doped mesoporous silica SBA-15, Sens. Actuators, B, 2008, 135, 255–261 CrossRef CAS.
- F. Maddox Sayler, M. G. Bakker, J.-H. Smått and M. Lindén, Correlation between electrical conductivity, relative humidity, and pore connectivity in mesoporous silica monoliths, J. Phys. Chem. C, 2010, 114, 8710–8716 CAS.
- J. Tu, R. Wang, W. Geng, X. Lai, T. Zhang, N. Li, N. Yue and X. Li, Humidity sensitive property of Li-doped 3D periodic mesoporous silica SBA-16, Sens. Actuators, B, 2009, 136, 392–398 CrossRef CAS.
- W. Geng, R. Wang, X. Li, Y. Zou, T. Zhang, J. Tu, Y. He and N. Li, Humidity sensitive property of Li-doped mesoporous silica SBA-15, Sens. Actuators, B, 2007, 127, 323–329 CrossRef CAS.
- W. Geng, L. Zhou, L. Duan, J. Gu, Q. Zhang, Q. Yuan and X. Jiang, Humidity sensing property of NaCl-added mesoporous silica synthesized by a facile way with low energy cost, Int. J. Appl. Ceram. Technol., 2015, 12, 169–175 CrossRef CAS.
- C. Pirez, J.-M. Caderon, J.-P. Dacquin, A. F. Lee and K. Wilson, Tunable KIT-6 mesoporous sulfonic acid catalysts for fatty acid esterification, ACS Catal., 2012, 2, 1607–1614 CrossRef CAS.
- M. Donohue and G. Aranovich, Classification of gibbs adsorption isotherms, Adv. Colloid Interface Sci., 1998, 76, 137–152 CrossRef.
- T. Zhang, R. Wang, W. Geng, X. Li, Q. Qi, Y. He and S. Wang, Study on humidity sensing properties based on composite materials of Li-doped mesoporous silica A-SBA-15, Sens. Actuators, B, 2008, 128, 482–487 CrossRef CAS.
- C.-T. Wang and C.-L. Wu, Electrical sensing properties of silica aerogel thin films to humidity, Thin Solid Films, 2006, 496, 658–664 CrossRef CAS.
- Y. M. Wang, Z. Y. Wu and J. H. Zhu, Surface functionalization of SBA-15 by the solvent-free method, J. Solid State Chem., 2004, 177, 3815–3823 CrossRef CAS.
- Q. Yuan, W. Geng, N. Li, J. Tu, R. Wang, T. Zhang and X. Li, Study on humidity sensitive property of K2CO3-SBA-15 composites, Appl. Surf. Sci., 2009, 256, 280–283 CrossRef CAS.
- L. Liu, L. Y. Kou, Z. C. Zhong, L. Y. Wang, L. F. Liu and W. Li, Preparation and humidity sensing properties of KCl/MCM-41 composite, Chin. Phys. Lett., 2010, 27, 050701 CrossRef.
- R. Wang, X. Liu, Y. He, Q. Yuan, X. Li, G. Lu and T. Zhang, The humidity-sensitive property of MgO-SBA-15 composites in one-pot synthesis, Sens. Actuators, B, 2010, 145, 386–393 CrossRef CAS.
- Q. Yuan, N. Li, J. Tu, X. Li, R. Wang, T. Zhang and C. Shao, Preparation and humidity sensitive property of mesoporous ZnO–SiO2 composite, Sens. Actuators, B, 2010, 149, 413–419 CrossRef CAS.
- M.-S. Gong, S.-W. Joo and B.-K. Choi, Humidity-sensitive properties of a cross-linked polyelectrolyte prepared from mutually reactive copolymers, J. Mater. Chem., 2002, 12, 902–906 RSC.
- L. Wang, F. Zhao, Q. Han, C. Hu, L. Lv, N. Chen and L. Qu, Spontaneous formation of Cu2O–gC3N4 core–shell nanowires for photocurrent and humidity responses, Nanoscale, 2015, 7, 9694–9702 RSC.
- A. D. Smith, K. Elgammal, F. Niklaus, A. Delin, A. C. Fischer, S. Vaziri, F. Forsberg, M. Råsander, H. Hugosson and L. Bergqvist, Resistive graphene humidity sensors with rapid and direct electrical readout, Nanoscale, 2015, 7, 19099–19109 RSC.
- M. E. Tuckerman, D. Marx and M. Parrinello, The nature and transport mechanism of hydrated hydroxide ions in aqueous solution, Nature, 2002, 417, 925–929 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03385g |
| This journal is © The Royal Society of Chemistry 2016 |





