A non-linear organic reaction of malonate derivative as a base amplifier to generate imidazoles without producing gas†
Abstract
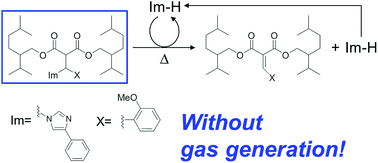
A malonate-type base amplifier that decomposes autocatalytically to generate imidazoles has been designed. This is the first example of a base amplifier that does not produce gas as a by-product.

 Please wait while we load your content...
Please wait while we load your content...