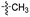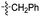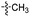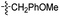Tuning the reaction rates of fluoride probes for detection in aqueous solution†
Yueqin Zheng‡
a,
Yuqing Duan‡ab,
Kaili Jia,
Run-Ling Wang*b and
Binghe Wang*a
aDepartment of Chemistry, Georgia State University, Atlanta, Georgia 30303-3083, USA. E-mail: wang@gsu.edu
bTianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy, Tianjin Medical University, Tianjin 300070, China. E-mail: wangrunling@tmu.edu.cn
First published on 2nd March 2016
Abstract
Fluoride detection in aqueous solution has drawn much attention. Most fluoride probes are based on the cleavage of a silyl group by fluoride for the generation of fluorescence. However, such a reaction is generally slow in aqueous solution. Herein we successfully demonstrate the concept that increasing the hydrophilicity of a pendent group enhances the reactivity of a silyl-based probe for fluoride detection in aqueous solution. By applying this concept, we also developed a new probe with a pendent PEG unit (BW-F-204), which showed excellent fluoride sensing ability both in aqueous solution and in cell culture.
Recently, a large number fluorescent sensors for anions have been reported.1–5 Fluoride is an important anion in toothpaste and drinking water. Trace amounts of fluoride in pharmaceutical agents or drinking water can be used to treat osteoporosis6–8 and prevent dental caries.9,10 However, excess amounts of fluoride could cause dental and bone fluorosis11 and other diseases such as gastric and kidney disorders and nephrorolithiasis.10 Therefore, fluoride detection in aqueous media has attracted much attention because of its potential applications in various areas.
Many fluorescent probes for fluoride ion have been developed. There are largely two strategies. One is based on reversible interactions involving hydrogen bond interactions,12,13 Lewis acid/base coordination,14 and anion-π interactions.15,16 Because fluoride is the smallest anion, its detection through complexation has been challenging, especially in aqueous solution in which fluoride is heavily solvated. The other method is to use an irreversible chemical reaction. The latter strategy has attracted much interest for its selectivity, simplicity, and potential applications in aqueous phase.16 Generally, the key design of these probes relies on the cleavage of either a C–Si17–20 or an O–Si16,21–25 bond by fluoride anion. This approach takes advantage of the high affinity of fluoride for a silyl group. Based on this principle, a number of chemosensors have been reported.1,26,27 Basically, these probes consist of two key moieties: a silylated phenol alcohol and a fluorescent reporter unit. Generally speaking, the silyl ether could be a tert-butyldiphenylsilyl (TBDPS), tert-butyldimethylsilyl (TBS/TBDMS) or triisopropylsilyl (TIPS) group, and the reporter unit is a fluorophore, which is sensitive to the availability of a free hydroxyl group in terms of fluorescence turn on. Among all the chemodosimeters, only a few of them can be applied in pure aqueous systems due to the slow reaction under such conditions.1,21,28–31 To enhance the probe's reactivity, organic solvents such as DMSO,16 THF32 and acetone18 are used. Sometimes co-solvent systems such as ACN/H2O33 and EtOH/H2O34 are used. Directly detecting fluoride anion in water is still a challenge, largely due to slow reactions between fluoride and the silyl ether in an aqueous environment. One way one could adjust the reaction rates is to make silyl ethers with low stability. However, such an approach affects the general stability and thus utility of the probes. We are interested in exploring other factors that can be tuned to improve performance of a fluoride probe in aqueous solution.
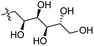
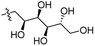
Our lab has had a long-standing interest in fluoride fluorescent probes. In 2013, we successfully investigated a benzothiazole-based fluoride sensor, which can be used in aqueous solution.21 Anecdotal evidence seems to suggest that the glucosamine group, which was used to help improve water solubility, might also enhance the desilylation reaction rate by fluoride in aqueous solution. This led us to systematically examine this issue with two series of silyl ether-based fluoride probes: one benzothiazole-based series (BW-F-101-103, Table 1) and another coumarin-based series (BW-F-201-203, Table 2). Specifically, in each series, we have three groups of varying hydrophilicity/hydrophobicity as pendent chains for water solubility modulation. In the benzothiazole series, BW-F-101 has a fluoride probe conjugated via an amide bond with a hydrophilic glucosamine; BW-F-102 a methylamine; and BW-F-103 a hydrophobic benzyl amine. These groups did not affect their excitation and emission significantly. Then we studied the reaction rates for these probes in aqueous solution. Specifically, we used 10 μM of the probes to react with 200 μM, 500 μM and 1 mM NaF in PBS, respectively. The fluorescence intensity changes were recorded to monitor the progression of the reaction. Then we calculated their concentration dependent pseudo first order rate constants, and subsequently the second order rate constants. From the results shown in Table 1, it is very clear that these pendent groups have a very significant effect on the reaction rate with fluoride in aqueous solution. The second order rate constant of BW-F-101 (0.54 M−1 s−1), which has a pendent glucosamine group, is about 65 fold larger than that of BW-F-103 (0.0083 M−1 s−1), which has a pendent hydrophobic benzyl group. The one that has a methyl group (BW-F-102) has a second order rate constant, which is somewhere in between (0.33 M−1 s−1). This is expected if water solubility is a key factor because BW-F-102 should have water solubility in between BW-F-101 and 103 as well. The results indicate that the water solubility difference introduced by the pendent groups seems to be directly correlated to the probe reactivity toward fluoride in aqueous solution.
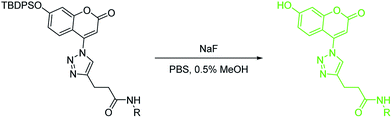
Next we studied the same issue using another series of fluoride probes based on the coumarin fluorophore. This is to make sure that the results obtained with the benzothiazole series was not due to idiosynchratic factors unique to the benzothiazole structural scaffold. Thus, we also synthesized coumarin-based fluoride probes (BW-F-201-203, Table 2). These probes were conjugated with the same pendent groups using click chemistry. Then we proceeded to studying the reaction kinetics in the same fashion as for the benzothiazole probes. The results obtained with the coumarin series corroborate the findings with the benzothiazole series. The probe with a pendent hydrophilic group, glucosamine (BW-F-201), showed a much faster reaction rate (56 fold) (second order rate constant: 0.56 M−1 s−1) than that of the one with a hydrophobic benzyl group (BW-F-203, 0.010 M−1 s−1). As expected, the one with a methyl group (BW-F-202) had a second order rate constant, which is somewhere in between (0.12 M−1 s−1). Such results with two series of fluorescent fluoride probes indeed point to water solubility being a critical factor in influencing the reaction rate of the probes with fluoride in aqueous solution. It should also be noted that the fluorescent intensity of these probes and their products after reaction with fluoride is linearly dependent on the concentration of the analytes (SI), indicating that aggregation is not a factor. Such findings should help efforts in designing future probes for detection of fluoride in aqueous solution.
As further efforts to validate and apply the idea of modulating a probe's reactivity through the introduction of a water-soluble group, we were interested in introducing a pendent polyethylene glycol unit to see whether such a strongly hydrophilic unit would help further improve reactivity. Therefore, we synthesized a polyethylene glycol (PEG, 1000 Da) conjugated fluoride probe BW-F-204. The introduction of the pendent PEG allowed BW-F-204 to be completely soluble in aqueous solution without the need for even a small amount of organic co-solvent. Then we also determined its second order rate constant using the same approach described, and found that BW-F-204 has the fasted reaction rate with a second order rate constant of 3.4 ± 0.2 M−1 s−1, which is 6-fold higher than that of the glucosamine conjugate (BW-F-201) and 340-fold faster than the one with a pendent benzyl group (BW-F-203). To further explore the general properties of this probe, we conducted selectivity studies by treating the probe (10 μM) with various anions (1 mM, AcO−, F−, Cl−, Br−, I−, HCO3−, HPO42−, N3−, NO2−, NO3−, SO32−, SO42−) for 30 min in PBS. Other than fluoride, none of the other anions led to any significant increases of fluorescent intensity. Such results indicate very good selectivity as expected with a silyl ether-based probe (Fig. 1).
Next we examined the sensitivity of the probe and its detection limit. Thus the probe was incubated with different concentrations of sodium fluoride in PBS for 15 min, and the changes of fluorescence intensity are shown in Fig. 2. The probe indeed showed concentration-dependent fluorescent changes. Using a signal to noise ratio of 3, the detection limit is about 18 μM, which is far below the EPA standard value (1.0 × 10−4 M) of recommended maximal concentration of fluoride in drinking water. Therefore, this probe can be directly used in the detection for fluoride anion for water quality monitoring.
 | ||
| Fig. 2 Fluorescence spectra of 50 μM of BW-F-204 in the presence of NaF at different concentrations (15 min at r.t.). | ||
We also wondered whether we could detect fluoride inside cells using BW-F-204, and thus conducted cell-imaging studies. Specifically, MB-231 cells were used and incubated with 0 μM, 20 μM, and 100 μM NaF for 30 min respectively. The media were then removed and the cells were further incubated with 10 μM BW-F-204 for 30 min. The cells were subsequently washed with PBS and fixed onto glass slides. Images were taken (Fig. 3). Strong fluorescence was detected in cells treated with 20 μM and 100 μM of NaF.
In conclusion, we synthesized two series of fluoride probes, and successfully demonstrated the concept that increasing the hydrophilicity of a pendent group can be used as a way to enhance the reactivity of probes for fluoride detection in aqueous solution. By applying this concept, we also developed one probe with a pendent PEG unit (BW-F-204), which showed excellent fluoride sensing ability both in solution and in cell culture. This probe can be directly used in aqueous solution. We hope that the findings presented will also help others in their pursuit of fluorescent probes for fluoride detection in aqueous solution. It will also be important to gather additional data to further examine whether the same concept holds true in other series of silyl-based fluoride probes.
Acknowledgements
We gratefully acknowledge a scholarship in support of YQD from the China Scholarship Council (No. 201406940013).Notes and references
- Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511–5571 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- K. Wang, H. Peng and B. Wang, J. Cell. Biochem., 2014, 115, 1007–1022 CrossRef CAS PubMed.
- P. Nandhikonda and M. D. Heagy, Org. Lett., 2010, 12, 4796–4799 CrossRef CAS PubMed.
- W. Jiang, Q. Fu, H. Fan, J. Ho and W. Wang, Angew. Chem., Int. Ed. Engl., 2007, 46, 8445–8448 CrossRef CAS PubMed.
- J. R. Farley, J. E. Wergedal and D. J. Baylink, Science, 1983, 222, 330–332 CAS.
- Z. Yang, K. Zhang, F. Gong, S. Li, J. Chen, J. S. Ma, L. N. Sobenina, A. I. Mikhaleva, G. Yang and B. A. Trofimov, Beilstein J. Org. Chem., 2011, 7, 46–52 CrossRef CAS PubMed.
- H. S. Horowitz, J. Publ. Health. Dent., 2003, 63, 3–8 CrossRef.
- M. A. Lennon, Bull. W. H. O., 2006, 84, 759–760 CrossRef PubMed.
- M. Kleerekoper, Endocrinol. Metab. Clin. North Am., 1998, 27, 441–452 CAS.
- S. Ayoob and A. K. Gupta, Crit. Rev. Environ. Sci. Technol., 2006, 36, 433–487 CrossRef CAS.
- M. Boiocchi, L. Del Boca, D. E. Gomez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed.
- K. J. Chang, D. Moon, M. S. Lah and K. S. Jeong, Angew. Chem., Int. Ed. Engl., 2005, 44, 7926–7929 CrossRef CAS PubMed.
- S. Yamaguchi, T. Shirasaka, S. Akiyama and K. Tamao, J. Am. Chem. Soc., 2002, 124, 8816–8817 CrossRef CAS PubMed.
- S. Guha, F. S. Goodson, L. J. Corson and S. Saha, J. Am. Chem. Soc., 2012, 134, 13679–13691, DOI:13610.11021/ja303173n.
- A. Roy, D. Kand, T. Saha and P. Talukdar, Chem. Commun., 2014, 50, 5510–5513 RSC.
- D. Buckland, S. V. Bhosale and S. J. Langford, Tetrahedron Lett., 2011, 52, 1990–1992 CrossRef CAS.
- L. Fu, F.-L. Jiang, D. Fortin, P. D. Harvey and Y. Liu, Chem. Commun., 2011, 47, 5503–5505 RSC.
- M. R. Rao, S. M. Mobin and M. Ravikanth, Tetrahedron, 2010, 66, 1728–1734 CrossRef CAS.
- H. Lu, Q. Wang, Z. Li, G. Lai, J. Jiang and Z. Shen, Org. Biomol. Chem., 2011, 9, 4558–4562 CAS.
- B. Ke, W. Chen, N. Ni, Y. Cheng, C. Dai, H. Dinh and B. Wang, Chem. Commun., 2013, 49, 2494–2496 RSC.
- S. Y. Kim, J. Park, M. Koh, S. B. Park and J.-I. Hong, Chem. Commun., 2009, 4735–4737 RSC.
- R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed. Engl., 2010, 49, 4915–4918 CrossRef CAS PubMed.
- O. A. Bozdemir, F. Sozmen, O. Buyukcakir, R. Guliyev, Y. Cakmak and E. U. Akkaya, Org. Lett., 2010, 12, 1400–1403 CrossRef CAS PubMed.
- X. Cao, W. Lin, Q. Yu and J. Wang, Org. Lett., 2011, 13, 6098–6101 CrossRef CAS PubMed.
- F. Zheng, F. Zeng, C. Yu, X. Hou and S. Wu, Chemistry, 2013, 19, 936–942 CrossRef CAS PubMed.
- T. H. Kim and T. M. Swager, Angew. Chem., Int. Ed. Engl., 2003, 42, 4803–4806 CrossRef CAS PubMed.
- G. Wei, J. Yin, X. Ma, S. Yu, D. Wei and Y. Du, Anal. Chim. Acta, 2011, 703, 219–225 CrossRef CAS PubMed.
- I. S. Turan and E. U. Akkaya, Org. Lett., 2014, 16, 1680–1683 CrossRef CAS PubMed.
- X. Zhou, R. Lai, H. Li and C. I. Stains, Anal. Chem., 2015, 87, 4081–4086 CrossRef CAS PubMed.
- L. Li, Y. Ji and X. Tang, Anal. Chem., 2014, 86, 10006–10009 CrossRef CAS PubMed.
- M. Dong, Y. Peng, Y.-M. Dong, N. Tang and Y.-W. Wang, Org. Lett., 2012, 14, 130–133 CrossRef CAS PubMed.
- P. Hou, S. Chen, H. Wang, J. Wang, K. Voitchovsky and X. Song, Chem. Commun., 2014, 50, 320–322 RSC.
- B. Zhu, F. Yuan, R. Li, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 7098–7100 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03252d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |