Development of an advanced chemical oxidation wastewater treatment system for the batik industry in Malaysia†
Abstract
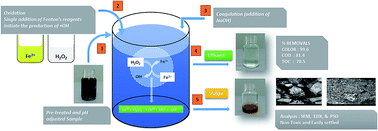
The batik industry is operated as a cottage industry due to variation in the designs and demand of batik. Batik is considered an art. However, the batik industry consumes a large volume of water and produces a large amount of wastewater that contains grease, resin, surfactants, wax, suspended solids and dyes. Hence, this industry requires treatment systems, which are inexpensive, simple, safe to operate, energy efficient, do not require skilled workers and do not produce secondary pollutants. In this regard, AOPs characterized by the production of highly reactive radicals, which are able to degrade most of the recalcitrant organic pollutants, have been found to fulfil the requirement. The Fenton process is one of the low-cost, low-footprint, simple and less complicated AOPs that can be used to treat effluent that has a high COD. At the optimal conditions (room temperature, undiluted contaminants), 81.4% COD, 70.5% TOC and 99.6% color removal were obtained within an hour by the Fenton process. The samples were analyzed using FTIR, GC-MS and HPLC, which confirmed the superiority of the Fenton process. The GC/MS analysis revealed that the Fenton process successfully removed 71% of organic compounds. Sludge characterization by SEM and particle size distribution showed that the Fenton generated sludge achieved suitable disposal qualities.


 Please wait while we load your content...
Please wait while we load your content...