SnSb/TiO2/C nanocomposite fabricated by high energy ball milling for high-performance lithium-ion batteries†
Abstract
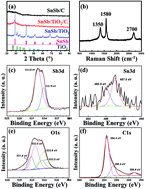
Alloy anodes for Li-ion batteries (LIBs) have attracted great interest due to their high capacity. However, their large volume change during electrochemical lithiation/delithiation causes a poor cycle life, which significantly limits their application. Here we design and fabricate a carbon-coated SnSb/TiO2 nanocomposite via an in situ mechanochemical reduction route, in which a nanostructured SnSb alloy is grown on TiO2 and coated by a layer of conductive carbon. Compared with the SnSb/TiO2 composite or C-coated SnSb alloy, such a C-coated SnSb/TiO2 nanocomposite (SnSb/TiO2/C) shows a higher reversible capacity of 630 mA h g−1 and better capacity retention (80% over 200 cycles). Our work suggests that the mechanochemical reduction by high energy ball milling can be a powerful method to fabricate alloy anodes with improved cycle stability for addressing the volume change issue.

 Please wait while we load your content...
Please wait while we load your content...