Helical supramolecular organization of a 1,2-diol appended naphthalene diimide organogelator via an extended intermolecular H-bonding network†
Sopan Valiba Shinde,
Mandar Kulkarni and
Pinaki Talukdar*
Department of Chemistry, Indian Institute of Science Education and Research Pune, India. E-mail: ptalukdar@iiserpune.ac.in; Fax: +91 20 2586 5086; Tel: +91 20 2590 8000
First published on 11th March 2016
Abstract
Self-assembly of a 1,2-diol appended naphthalene diimide derivative featuring chiral and J-type aggregation is reported. In MCH/CHCl3, the compound exhibited intense yellow excimer and thermoreversible “sol–gel” behavior. Morphological and circular dichroism studies revealed long range M-helical nanofibre formation. A theoretical model of the cooperative hydrogen bonding was also proposed.
Self-assembly of small molecular building blocks is one of the classical strategies for designing various functional nanoscale architectures.1–3 Over the past two decades, incommensurable supramolecular assemblies of small organic molecules were explored utilizing noncovalent interactions.4 The hierarchical self-assembly of different chromophoric π-systems has unfolded diverse applications in chemistry, material science, and biology.5–7 Among the class of rylene diimide dyes,8 1,4,5,8-naphthalene diimides (NDIs) have been utilized extensively as critical units for constituting smart supramolecular nanostructures such as catenanes,9,10 rotaxanes,11–14 foldamers,15–18 molecular knots,19 vesicles,20 ion channels,21 nanotubes,22 hydrogels,23,24 organogels,25,26 etc. Longitudinally self-assembled NDIs from small molecules and polymers are also attractive n-type materials for organic electronic devices, photosystems,27,28 and solar cells.29,30 Other noncovalent interactions such as, hydrogen bonding (from carboxylic acids31,32 and amides20,33) van der Waals interactions,34 etc. contribute synergistically to the ordered longitudinal orientation of these π-conjugated building blocks.
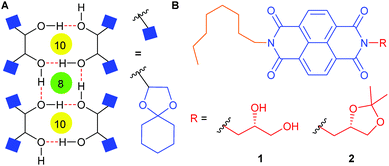
Recently, Ghosh and coworkers have reported a unique achiral extended self-assembly of NDIs based on simultaneous intra and inter-molecular hydrogen bonding motifs of tethered 1,3-dihydroxyl moieties.35 The study paved the new ground in investigating the NDI aggregation based on hydrogen bonding of aliphatic hydroxyl groups. Solid state self-assembly of diketal protected mannitol derivatives with free 1,2-dihydroxyl groups was reported by us disclosing an extended network of alternate eight- and ten-membered rings through intermolecular hydrogen bond interactions (Fig. 1A).36 Herein, we report a 1,2-dihydroxyl functionalized NDI amphiphile 1 (Fig. 1B) that provides a chiral and stable self-assembly through hydrogen bonding interactions palpably akin to those of the mannitol derivatives. The present work also features atypical red shifted NDI excimer emission and thermoresponsive gelation behavior of 1. A control NDI derivative 2 was also designed to address the importance of hydrogen bonding interactions in the self-assembly process.
In CHCl3, compound 1 (see ESI† for the preparation of 1 and 2) exhibited well resolved π–π* absorption bands at 342, 361 and 381 nm indicating the monomeric state of the NDI chromophore (Fig. 2A). The change of solvent to nonpolar methyl cyclohexane (MCH) resulted in the considerable reduction of monomeric absorbance along with the concomitant 15 nm red-shifted absorption maxima indicating a J-type π–π aggregation state of NDI moieties.37 The steady-state emission spectrum of 1 in CHCl3 also provided characteristic monomeric signals at 387, 408 and 432 nm (Fig. 2B). The same compound when recorded in MCH, displayed red-shifted emission at 400–500 nm (a signature of vibrational progression),38 and a strong excimer band centered at 578 nm. Such a red-shifted NDI excimer emission corroborates to strong inter-chromophoric interactions during the self-assembly process. The excitation spectrum collected at 578 nm displayed red-shifted excitation maxima as compared to monomeric absorption band confirming excimer emission is from J-aggregation of NDI chromophores (Fig. S5†). The ketal protected compound 2 displayed nearly identical UV-visible spectra in CHCl3 and MCH. These results indicate that the observed spectral differences for 1 in these media are not caused by mere solvent effects. Fluorescence spectra of 2 in these two solvents were also comparable. This study confirms that the self-assembly of 1 in MCH is assisted predominately by hydrogen bonding interactions among hydroxyl groups leading to the aggregation induced enhanced emission (AIEE).39
Further, to probe the effect of solvent composition on self-assembly, absorption spectra of 1 were recorded in variable solvent ratio of MCH and CHCl3 (Fig. 2C). Up to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 MCH/CHCl3, the absorption spectra resembled that recorded in pure CHCl3. A stepwise decrease of absorption bands at 338, 356 and 381 nm with the appearance of new absorption band at 402 nm wavelength was observed above 70
30 MCH/CHCl3, the absorption spectra resembled that recorded in pure CHCl3. A stepwise decrease of absorption bands at 338, 356 and 381 nm with the appearance of new absorption band at 402 nm wavelength was observed above 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 MCH/CHCl3. However, the change of solvent from 95
30 MCH/CHCl3. However, the change of solvent from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 to pure MCH led to a 7 nm blue-shift of the absorption band suggesting a minor effect due to solvent polarity. The increase of MCH percentage in CHCl3 also provided a considerable change in the emission spectrum of 1 (Fig. 2D). The monomeric behavior of 1 was evident till 70
5 MCH/CHCl3 to pure MCH led to a 7 nm blue-shift of the absorption band suggesting a minor effect due to solvent polarity. The increase of MCH percentage in CHCl3 also provided a considerable change in the emission spectrum of 1 (Fig. 2D). The monomeric behavior of 1 was evident till 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 MCH/CHCl3. Further increase of MCH percentage resulted in the decrease of characteristic monomer signals and increase of aggregation bands. The appearance of the excimer around 560 nm was first observed in 75
30 MCH/CHCl3. Further increase of MCH percentage resulted in the decrease of characteristic monomer signals and increase of aggregation bands. The appearance of the excimer around 560 nm was first observed in 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 MCH/CHCl3. In 85
25 MCH/CHCl3. In 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 MCH/CHCl3, the intensity of the excimer band increased and a shoulder band at 596 nm was observed. Further decrease in the solvent polarity to 95
15 MCH/CHCl3, the intensity of the excimer band increased and a shoulder band at 596 nm was observed. Further decrease in the solvent polarity to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 resulted in an averaged out excimer band at 578 nm. To understand the excited state dynamics of 1, time-correlated single-photon counting (TCSPC) experiments were performed with excitation at 340 nm (Fig. S8†). The emission monitored at 578 nm decayed bi-exponentially (τ1 = 6.94 ns (30.54%), τ2 = 18.33 ns (69.46%)). The emission monitored at 447 nm decayed mono-exponentially (τ = 1.02 ns). These longer lived decays are characteristics of J-aggregated strong intermolecular chromophoric interactions.23,24
5 MCH/CHCl3 resulted in an averaged out excimer band at 578 nm. To understand the excited state dynamics of 1, time-correlated single-photon counting (TCSPC) experiments were performed with excitation at 340 nm (Fig. S8†). The emission monitored at 578 nm decayed bi-exponentially (τ1 = 6.94 ns (30.54%), τ2 = 18.33 ns (69.46%)). The emission monitored at 447 nm decayed mono-exponentially (τ = 1.02 ns). These longer lived decays are characteristics of J-aggregated strong intermolecular chromophoric interactions.23,24
To prove the importance of hydrogen bonding in the aggregation of 1, absorption spectra of 1 were recorded with increasing percentage of MeOH in MCH. In these experiments, the signal at 396 nm displayed a 5 nm red-shift however, with a decrease in absorbance (Fig. S7A†). The addition of MeOH also resulted in the increase of signals corresponding to monomeric structure. A plot of maximum absorbance around 396–401 nm versus MeOH percentage indicated a complete disaggregation of around 2.6% of the protic solvent (Fig. S7B†). This study suggests that the longitudinal π-stacked self-assembly of 1 is sustained predominantly by intermolecular hydrogen bonding interactions among vicinal hydroxyl groups of 1. Based on the aforestated self-assembly, gelation behavior of the compound was explored in various solvent systems (Table S1†). In MCH/CHCl3 solvent system at room temperature, 1 (1.5 mg mL−1) formed white suspension which upon heating provided clear solution. However slow cooling of solution did not provide any gel formation below 60% of the less polar solvent. Interestingly, the stable gel formation was observed in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 MCH/CHCl3 (Fig. 3A). The gel also displayed thermoreversible “sol–gel” behavior up to five heating–cooling cycles. The preformed gel was also stable even after four months upon storing at room temperature. When placed under the hand-held UV-lamp (λex = 364 nm), the solution of 1 in CHCl3 provided weak blue fluorescence while that in 95
30 MCH/CHCl3 (Fig. 3A). The gel also displayed thermoreversible “sol–gel” behavior up to five heating–cooling cycles. The preformed gel was also stable even after four months upon storing at room temperature. When placed under the hand-held UV-lamp (λex = 364 nm), the solution of 1 in CHCl3 provided weak blue fluorescence while that in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 exhibited strong yellow fluorescence (Fig. 3B). The gel formed in 70
5 MCH/CHCl3 exhibited strong yellow fluorescence (Fig. 3B). The gel formed in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 MCH/CHCl3 also provided a similar yellow fluorescence (Fig. 3C) indicating a comparable self-assembly in 95
30 MCH/CHCl3 also provided a similar yellow fluorescence (Fig. 3C) indicating a comparable self-assembly in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 and in the gel. Table S1† also indicates the gel formation in other non-polar organic solvents. It is noteworthy that neither the gel formation in 95
5 MCH/CHCl3 and in the gel. Table S1† also indicates the gel formation in other non-polar organic solvents. It is noteworthy that neither the gel formation in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 was affected, nor the preformed gel was disturbed upon addition of pyrene. These results suggest that the self-assembly of the 1 is rather strong and the aggregation does not allow interdigitation of electron rich pyrene within the π-stacked NDI layers. Additionally, UV-visible spectrum of 1 in the presence of pyrene did not indicate any charge transfer band formation and this result is also supportive of strong NDI self-assembly.
5 MCH/CHCl3 was affected, nor the preformed gel was disturbed upon addition of pyrene. These results suggest that the self-assembly of the 1 is rather strong and the aggregation does not allow interdigitation of electron rich pyrene within the π-stacked NDI layers. Additionally, UV-visible spectrum of 1 in the presence of pyrene did not indicate any charge transfer band formation and this result is also supportive of strong NDI self-assembly.
To understand the morphology of self-assembly of 1, field-emission scanning electron microscopic (FESEM), transmission electron microscopic (TEM) and atomic force microscopic (AFM) data were recorded by drop casting a MCH suspension of the gel on solid surface. All these experiments confirmed the formation of left-handed (M) helical (helix pitch ∼126 nm) nanofibres of considerable length with variable entanglement (Fig. 4). FESEM and AFM data recorded by drop casting 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 solution (Fig. S9†) of 1 also showed comparable left-handed helical nanofibres confirming the similarity of self-assembled morphology in the gel and 95
5 MCH/CHCl3 solution (Fig. S9†) of 1 also showed comparable left-handed helical nanofibres confirming the similarity of self-assembled morphology in the gel and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 solution. The long-range orientation of 1 within the self-assembled nanofiber was investigated using the circular dichroism (CD) spectroscopy. The CD spectrum of 1 displayed a flat profile in CHCl3, whereas the same molecule when recorded in 95
5 MCH/CHCl3 solution. The long-range orientation of 1 within the self-assembled nanofiber was investigated using the circular dichroism (CD) spectroscopy. The CD spectrum of 1 displayed a flat profile in CHCl3, whereas the same molecule when recorded in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MCH/CHCl3 revealed a positive exciton couplet at 248 nm along with strong excitonic negative Cotton effect at 401 nm. The bisignated nature of CD curve indicates the strong excitonic coupling between NDI chromophores40 and the long wavelength negative CD signal corresponds to the distinctive π–π* transition along the long axis of NDIs self-assembled in the M-helical arrangement.22,41–44 (Fig. S10†). The observed left-handed chiral sense is induced by the synergistic effect of chirality transfer from the chiral diol linkage and steric congestion among alkyl chains in the fibrillar structure.45,46
5 MCH/CHCl3 revealed a positive exciton couplet at 248 nm along with strong excitonic negative Cotton effect at 401 nm. The bisignated nature of CD curve indicates the strong excitonic coupling between NDI chromophores40 and the long wavelength negative CD signal corresponds to the distinctive π–π* transition along the long axis of NDIs self-assembled in the M-helical arrangement.22,41–44 (Fig. S10†). The observed left-handed chiral sense is induced by the synergistic effect of chirality transfer from the chiral diol linkage and steric congestion among alkyl chains in the fibrillar structure.45,46
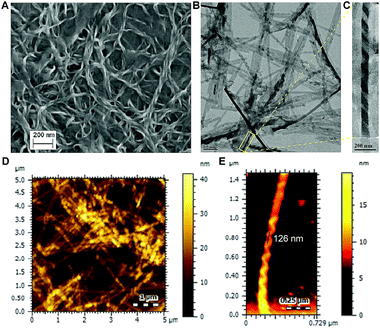 | ||
| Fig. 4 FESEM (A), TEM (B) and AFM (D) images recorded after drop-casting the MCH suspension of the gel prepared from 1. Expanded regions of TEM (C) and AFM (E) images are also shown. | ||
The X-ray diffraction study on the xerogel also provided the crucial insight about the self-assembled gel state. In the low angle region, sharp reflections at 2θ = 2.55° (d = 34.56 Å), 5.09° (d = 17.35 Å), and 7.50° (d = 11.55 Å) (i.e. at reciprocal spacing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) demonstrating formation of strong lamellar type of packing in the gel state (Fig. S11A and B†).47 The reflection at 5.09° (d = 17.35 Å) can also be correlated to the length of the monomer 1 (Fig. S15C†). In addition to this, the reflection at 2θ = 2.55° (d = 34.56 Å) can be correlated to a tail-to-tail distance of the 1-dimer preferably connected by hydrogen bonding interactions of hydroxyl groups. Reflections at 8.68 Å (D/4), 6.94 Å (D/5), 5.78 Å (D/6) and 4.33 Å (D/8) also support the lamellar structures in the self-assembled gel state.26 Furthermore, a peak at d = 3.46 Å (2θ = 25.727°) was assigned as the π–π stacking distance between two adjacent NDI units.48
1) demonstrating formation of strong lamellar type of packing in the gel state (Fig. S11A and B†).47 The reflection at 5.09° (d = 17.35 Å) can also be correlated to the length of the monomer 1 (Fig. S15C†). In addition to this, the reflection at 2θ = 2.55° (d = 34.56 Å) can be correlated to a tail-to-tail distance of the 1-dimer preferably connected by hydrogen bonding interactions of hydroxyl groups. Reflections at 8.68 Å (D/4), 6.94 Å (D/5), 5.78 Å (D/6) and 4.33 Å (D/8) also support the lamellar structures in the self-assembled gel state.26 Furthermore, a peak at d = 3.46 Å (2θ = 25.727°) was assigned as the π–π stacking distance between two adjacent NDI units.48
Several attempts of NDI 1 crystallization from different solvent systems were unsuccessful. Thus, to obtain more structural insight of the observed self-assembled helical fibrillar packing, computational studies at semi-empirical as well as DFT level were performed. The calculations were done using Gaussian 09 (ref. 49) and MOPAC 201250 software. The initial geometry of the hydrogen bonded system M2,2 was generated by placing two dimer M2 units on each other at a stacking distance of 4.68 Å to avoid intermolecular steric clashes (Fig. S14†). Geometry optimization of the M2,2 system at semi-empirical (Fig. S15A†) and DFT levels (Fig. S15B†) resulted near identical stacking distances of ∼3.4 Å. Subsequently, the octameric complex 12,8 was built by placing head-to-head hydrogen bonded dimer of 1 at a 4.68 Å distance above the original and rotating by 3° in the clockwise direction. The 12,8 complex was optimized using PM6-DH2 method available in MOPAC2012 software. The hydrogen bonds were not restrained during optimization of the octameric complex. The optimized structure obtained using PM6-DH2 method is well stabilized by hydrogen bond interactions of hydroxyl groups (i.e. via the network of alternate eight- and ten-membered hydrogen bonded rings),36 offset π-stacking and dispersion interactions (Fig. 5A and S17†). Interestingly, the calculated stacking distance of 3.39 Å closely resembled that obtained from the PXRD data (3.46 Å). These results suggest that the strong chromophoric interactions may be responsible for red-shifted excimer emission. The theoretically calculated tail-to-tail distance (41.78 Å) was somewhat longer than that obtained from the PXRD study (34.56 Å). This observation along with SEM images (thickness of fibres = 10–100 nm) imply that the monomer units are involved in repetitive tail-to-tail self-assembly with interdigitation of alkyl chains,51 and this leads to further aggregation into thick fibres.
Therefore, a plausible mechanism of the self-assembly process is proposed based on the above studies. At first, the dimerization of NDI 1 through head-to-head hydrogen bonding gives 12 which upon supramolecular polymerization leads to the fibril 12,n with the M-helical arrangement (Fig. 5B). Further bundling of these fibrils forms thicker fibres (i.e. via tail-to-tail interactions of aliphatic chains).
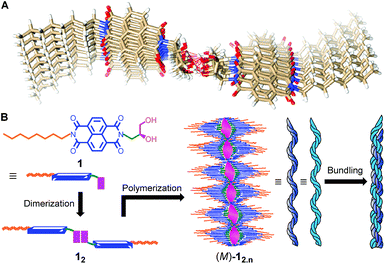 | ||
| Fig. 5 Top view of the geometry optimized (PM6-DH2) structure of 12,8 (A). Schematic representation of the self-assembly process (B). | ||
The presence of extended π-stacked NDI self-assembly endowed us to examine conducting behavior of 1. The charge carrier mobility was evaluated by the current–voltage (I–V) measurement under inert gas atmosphere. The diode current–voltage (J–V) curve shows the deviation from the Ohm's law at the high voltage (Fig. S12†) indicating semiconducting nature of 1.
In conclusion, we have demonstrated M-helical J-type aggregation of naphthalene diimides upon appending a polar 1,2-dihydroxyl moiety and a long hydrophobic chain at two imide termini. Photoluminescence study showed the atypical red-shifted strong excimer emission in 95% MCH. Morphological and circular dichroism (CD) studies confirmed fibrous nature with the M-helical arrangement which is responsible for gelation from a non-polar organic solvent with very low critical gel concentration. An extended hydrogen bonded network of alternate eight- and ten-membered rings involving 1,2-dihydroxyl groups was proposed for the self-assembly. Hydrogen bonding, offset π-stacking, dispersion interactions and chirality of the vicinal diol moiety participate synergistically to ensure M-helical self-assembly with strong NDI chromophoric interactions.
Acknowledgements
This work was supported in part by grants from DST under SERB scheme (SR/S1/OC-65/2012 and SB/S1/PC-39/2012). S. V. S. thanks CSIR and M. K. thanks IISER Pune for research fellowships. We thank Dr K. Krishnamoorthy of NCL, Pune for the recording of conductivity data, Dr A. Mukherjee and Dr N. Ballav of IISER Pune for their suggestions and comments.Notes and references
- E. Moulin, E. Busseron and N. Giuseppone, in Supramolecular Materials for Opto-Electronics, The Royal Society of Chemistry, 2015, pp. 1–52 Search PubMed.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- D. González-Rodríguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310 CrossRef.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS PubMed.
- J. Chen and F. Cheng, Acc. Chem. Res., 2009, 42, 713 CrossRef CAS PubMed.
- L. Cademartiri and G. A. Ozin, Adv. Mater., 2009, 21, 1013 CrossRef CAS.
- J. M. Warman, M. P. de Haas, G. Dicker, F. C. Grozema, J. Piris and M. G. Debije, Chem. Mater., 2004, 16, 4600 CrossRef CAS.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed.
- A. C. Try, M. M. Harding, D. G. Hamilton and J. K. M. Sanders, Chem. Commun., 1998, 723 RSC.
- L. Raehm, D. G. Hamilton and J. K. M. Sanders, Synlett, 2002, 1743 CAS.
- H. Y. Au-Yeung, G. Dan Pantos and J. K. M. Sanders, J. Am. Chem. Soc., 2009, 131, 16030 CrossRef CAS PubMed.
- K. M. Mullen, J. J. Davis and P. D. Beer, New J. Chem., 2009, 33, 769 RSC.
- H. Y. Au-Yeung, G. D. Pantos and J. K. M. Sanders, Angew. Chem., Int. Ed., 2010, 49, 5331 CrossRef CAS PubMed.
- H. Wilson, S. Byrne, N. Bampos and K. M. Mullen, Org. Biomol. Chem., 2013, 11, 2105 CAS.
- S. De and S. Ramakrishnan, Chem.–Asian J., 2011, 6, 149 CrossRef CAS PubMed.
- J. J. Reczek, K. R. Villazor, V. Lynch, T. M. Swager and B. L. Iverson, J. Am. Chem. Soc., 2006, 128, 7995 CrossRef CAS PubMed.
- R. S. Lokey and B. L. Iverson, Nature, 1995, 375, 303 CrossRef CAS.
- A. J. Zych and B. L. Iverson, J. Am. Chem. Soc., 2000, 122, 8898 CrossRef CAS.
- N. Ponnuswamy, F. B. L. Cougnon, J. M. Clough, G. D. Pantos and J. K. M. Sanders, Science, 2012, 338, 783 CrossRef CAS PubMed.
- M. R. Molla and S. Ghosh, Chem.–Eur. J, 2012, 18, 9860 CrossRef CAS PubMed.
- P. Talukdar, G. Bollot, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2005, 127, 6528 CrossRef CAS PubMed.
- H. Shao, M. Gao, S. H. Kim, C. P. Jaroniec and J. R. Parquette, Chem.–Eur. J., 2011, 17, 12882 CrossRef CAS.
- H. Shao, T. Nguyen, N. C. Romano, D. A. Modarelli and J. R. Parquette, J. Am. Chem. Soc., 2009, 131, 16374 CrossRef CAS PubMed.
- H. Shao and J. R. Parquette, Chem. Commun., 2010, 46, 4285 RSC.
- P. Mukhopadhyay, Y. Iwashita, M. Shirakawa, S.-i. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1592 CrossRef CAS PubMed.
- S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2013, 49, 6891 RSC.
- S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84 CrossRef CAS PubMed.
- R. Bhosale, J. Misek, N. Sakai and S. Matile, Chem. Soc. Rev., 2010, 39, 138 RSC.
- H. E. Katz, A. J. Lovinger, J. Johnson, C. Kloc, T. Slegrist, W. Li, Y. Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478 CrossRef CAS PubMed.
- S. V. Bhosale, C. H. Jani and S. J. Langford, Chem. Soc. Rev., 2008, 37, 331 RSC.
- M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Chem.–Eur. J., 2014, 20, 760 CrossRef CAS PubMed.
- A. Datar, K. Balakrishnan and L. Zang, Chem. Commun., 2013, 49, 6894 RSC.
- M. R. Molla and S. Ghosh, Chem. Mater., 2011, 23, 95 CrossRef CAS.
- K. Liu, C. Wang, Z. Li and X. Zhang, Angew. Chem., Int. Ed., 2011, 50, 4952 CrossRef CAS PubMed.
- T. Mondal, D. Basak, A. Al Ouahabi, M. Schmutz, P. Mesini and S. Ghosh, Chem. Commun., 2015, 51, 5040–5043 RSC.
- T. Saha, S. Dasari, D. Tewari, A. Prathap, K. M. Sureshan, A. K. Bera, A. Mukherjee and P. Talukdar, J. Am. Chem. Soc., 2014, 136, 14128 CrossRef CAS PubMed.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed.
- T. Okazaki, Y. Iizumi, S. Okubo, H. Kataura, Z. Liu, K. Suenaga, Y. Tahara, M. Yudasaka, S. Okada and S. Iijima, Angew. Chem., Int. Ed., 2011, 50, 4853 CrossRef CAS PubMed.
- S. S. Babu, K. K. Kartha and A. Ajayaghosh, J. Phys. Chem. Lett., 2010, 1, 3413 CrossRef CAS.
- M. Kumar, N. Jonnalagadda and S. J. George, Chem. Commun., 2012, 48, 10948 RSC.
- M. Kumar, S. J. George, P. Brocorens, C. Tonnele, D. Beljonne and M. Surin, Nat. Commun., 2014, 5, 5793 CrossRef CAS PubMed.
- M. Pandeeswar, M. B. Avinash and T. Govindaraju, Chem.–Eur. J., 2012, 18, 4818 CrossRef CAS PubMed.
- B. Narayan, C. Kulkarni and S. J. George, J. Mater. Chem. C, 2013, 1, 626 RSC.
- Z. Liu, G. Liu, Y. Wu, D. Cao, J. Sun, S. T. Schneebeli, M. S. Nassar, C. A. Mirkin and J. F. Stoddart, J. Am. Chem. Soc., 2014, 136, 16651 CrossRef CAS PubMed.
- K.-H. Ernst, in Supramolecular Chirality, ed. M. Crego-Calama and D. Reinhoudt, Springer, Berlin Heidelberg, 2006, vol. 265, pp. 209–252 Search PubMed.
- V. C. Edelsztein, A. S. Mac Cormack, M. Ciarlantini and P. H. Di Chenna, Beilstein J. Org. Chem., 2013, 9, 1826 CrossRef PubMed.
- M. K. Nayak, B.-H. Kim, J. E. Kwon, S. Park, J. Seo, J. W. Chung and S. Y. Park, Chem.–Eur. J., 2010, 16, 7437 CrossRef CAS PubMed.
- M. Tomasulo, D. M. Naistat, A. J. P. White, D. J. Williams and F. M. Raymo, Tetrahedron Lett., 2005, 46, 5695 CrossRef CAS.
- M. J. Frisch, et al., GAUSSIAN 09, Revision C.01, Gaussian, Inc, Wallingford CT, 2010 Search PubMed.
- J. J. P. Stewart, MOPAC2012, Stewart Computational Chemistry, Colorado Springs, CO, USA, 2012 Search PubMed.
- S. Yagai, H. Aonuma, Y. Kikkawa, S. Kubota, T. Karatsu, A. Kitamura, S. Mahesh and A. Ajayaghosh, Chem.–Eur. J., 2010, 16, 8652 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, supplemental data, and the 1H-, 13C-NMR spectrum. See DOI: 10.1039/c6ra02729f |
| This journal is © The Royal Society of Chemistry 2016 |