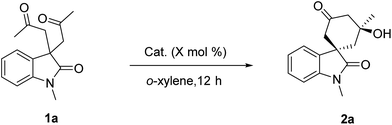
Dinuclear zinc-catalyzed desymmetric intramolecular aldolization: an enantioselective construction of spiro[cyclohexanone-oxindole] derivatives†
Ya-Fei Zhang,
Shao-Jie Yin,
Min Zhao,
Jun-Qi Zhang,
Hai-Yan Li and
Xing-Wang Wang*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: wangxw@suda.edu.cn; Fax: +86-512-6588-0378
First published on 10th March 2016
Abstract
Based on oxindole-derived diketones as the substrates, asymmetric desymmetrizing intramolecular aldol or aldol condensation reactions are reported, which are catalyzed by a Trost bis-ProPhenol dinuclear zinc complex. The corresponding spiro[cyclohexanone-oxindole] derivatives were obtained in good yields with moderate to good enantioselectivities.
Introduction
The aldol or aldol condensation, which provides practical β-hydroxycarbonyl skeleton or α,β-unsaturatured carbonyl compounds, is one type of classic carbon–carbon bond formation reaction and has been developed into an indispensable and efficient tool in organic synthesis.1 Numerous catalysts of the aldol reaction, including enzymes,2 metal complexes3 and small molecules,4 have been reported. The mechanism of some metal-catalyzed direct asymmetric aldol reactions imitates that of fuculose-1-phosphate aldolase (type II aldolase), which employs a zinc ion to acidify the α-proton of the donor component to form an enolate.5 Inspired by the catalysis of fuculose-1-phosphate aldolase, in 2000, Trost and Ito reported a dinuclear zinc catalyst, produced by the use of a ligand known as ProPhenol, for the aldol reaction of aryl methyl ketones with a wide variety of aldehydes with excellent catalytic outcomes.6The asymmetric desymmetrization of meso compounds by enzymatic7 and nonenzymatic8 methods has proven to be a versatile and powerful strategy in asymmetric synthesis. Asymmetric desymmetrization of diketones was initially reported via organocatalysis by Agami et al. in 1984, albeit the reaction led to cyclohexenone derivatives only with moderate enantioselectivities and in poor yields.9 Subsequently, Lerner et al. found that antibody 38C2 was a more active enzymatic catalyst, but the enantioselectivity remained moderate.10 In 2008, List et al. solved the longstanding problem of enantioselective desymmetrizing aldolizations of 4-substituted 2,6-heptanediones to enantiomerically enriched 5-substituted 3-methyl-2-cyclohexene-1-ones, in which Cinchona alkaloid deriving a primary amine in combination with acetic acid turned out to be efficient and highly enantioselective catalysts to furnish corresponding aldol condensation products in good yields with high enantioselectivities.11
The spirooxindoles represent a large family of alkaloids, natural products and pharmaceutically relevant compounds, which display remarkable structural complexity and interesting biological activity.12 Consequently, various synthetic protocols have been developed over the past few decades for the construction of multistereogenic spirocyclic oxindoles.13 In particular, the spirocyclic oxindole family with a chiral spiro[cyclohexanone-1,3′-indoline] core, an intriguing combination of multistereogenic cyclohexanone and oxindole motifs, is a promising subset with potential bioactivity.14 In 2009, Melchiorre and co-workers pioneered the syntheses of spiro[cyclohexane-1,3′-indoline]-2′,4-diones via [4 + 2] double Michael additions.15 Gong et al.,16 Wang et al.,17 and our group18 each reported highly enantioselective syntheses of spiro[cyclohexanone-oxindole] or spiro[cyclohexenone-oxindole] derivatives through organocatalytic cascade transformations. Herein, we report our preliminary results on a desymmetrizing intramolecular aldol reaction for asymmetric synthesis of spiro[cyclohexanone-oxindole] compounds in high yields with moderate to good enantioselectivities.
Results and discussion
Initially, a series of dinuclear zinc catalysts were prepared in situ by the addition of a solution of diethylzinc into a solution of chiral ligands L1–9 (Scheme 1) in o-xylene according to the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature. Then, these catalysts with 10 mol% catalyst loading were respectively employed in the asymmetric intramolecular aldolization of diketone oxindole 1a in the presence 30 mg of 4 Å molecular sieve (MS) in o-xylene at room temperature. As shown in Table 1, the ligands (R,R)-L1, (R)-L2, (S,S)-L3 and (S,S)-L4 were found to be ineffective for the reaction, affording essentially only trace amounts of the desired products (Table 1, entries 1–4). Subsequently, we tested Trost's dinuclear zinc catalysts for this transformation. The bis-ProPhenol ligands (S,S)-L5–9 were varied with different substituents on (S)-prolinol backbone. The screening results indicated that bis-ProPhenol ligand (S,S)-L9 was the best choice with 30 mg of 4 Å MS in the catalyst solution at room temperature for 12 hours, which provided the desired product 2a in 80% yield and 1
1 at room temperature. Then, these catalysts with 10 mol% catalyst loading were respectively employed in the asymmetric intramolecular aldolization of diketone oxindole 1a in the presence 30 mg of 4 Å molecular sieve (MS) in o-xylene at room temperature. As shown in Table 1, the ligands (R,R)-L1, (R)-L2, (S,S)-L3 and (S,S)-L4 were found to be ineffective for the reaction, affording essentially only trace amounts of the desired products (Table 1, entries 1–4). Subsequently, we tested Trost's dinuclear zinc catalysts for this transformation. The bis-ProPhenol ligands (S,S)-L5–9 were varied with different substituents on (S)-prolinol backbone. The screening results indicated that bis-ProPhenol ligand (S,S)-L9 was the best choice with 30 mg of 4 Å MS in the catalyst solution at room temperature for 12 hours, which provided the desired product 2a in 80% yield and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr, 97% ee (Table 1, entry 9 vs. entries 1–8). When 3 Å MS or 13 Å MS was introduced into the reaction, or without MS, poorer catalytic results were observed (Table 1, entries 10–12).
10 dr, 97% ee (Table 1, entry 9 vs. entries 1–8). When 3 Å MS or 13 Å MS was introduced into the reaction, or without MS, poorer catalytic results were observed (Table 1, entries 10–12).
| Entrya | Ligand | Additive | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1a (0.1 mmol), L (10 mol%), ZnEt2 (20 mol%), and 30 mg MS in o-xylene (1 mL) for 12 hours at room temperature (30 °C).b Isolated yield.c Determined by 1H NMR analysis.d Determined by chiral HPLC analysis (Chiralpak AD-H). | |||||
| 1 | L1 | 4 Å MS | 18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−15 |
| 2 | L2 | 4 Å MS | 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
29 |
| 3 | L3 | 4 Å MS | 15 | 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0/0 |
| 4 | L4 | 4 Å MS | <10 | — | — |
| 5 | L5 | 4 Å MS | <10 | — | — |
| 6 | L6 | 4 Å MS | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
51 |
| 7 | L7 | 4 Å MS | 15 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
| 8 | L8 | 4 Å MS | <10 | — | — |
| 9 | L9 | 4 Å MS | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
97 |
| 10 | L9 | 3 Å MS | 71 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 2.9 |
93 |
| 11 | L9 | 13 Å MS | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 2.9 |
90 |
| 12 | L9 | None | 75 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
85 |
Further results on the optimization of other parameters for the intramolecular aldol reaction catalyzed by dinuclear zinc complex ZnEt2/(S,S)-L9, including reaction medium, catalyst loading and reaction temperature, are summarized in Table 2. Firstly, we found that the reactivity was greatly influenced by the reaction medium (Table 2, entries 1–6). By the use of toluene, o-xylene or diethyl ether as the reaction medium, the reactions proceeded very well to furnish the desired products in 85–90% yields with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–1
9–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr and 94–97% ee (Table 2, entries 1–3). In comparison, the reactions proceeded in an obviously sluggish manner in m-xylene, THF and CH2Cl2, which afforded the desired products in lower yields and with lower ee (Table 2, entries 4–6). In addition, when the reaction temperature was decreased from 30 °C to 20 °C, the diastereoselectivity was dramatically reduced to 1
10 dr and 94–97% ee (Table 2, entries 1–3). In comparison, the reactions proceeded in an obviously sluggish manner in m-xylene, THF and CH2Cl2, which afforded the desired products in lower yields and with lower ee (Table 2, entries 4–6). In addition, when the reaction temperature was decreased from 30 °C to 20 °C, the diastereoselectivity was dramatically reduced to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.3 (Table 2, entry 7). When increasing the reaction temperature to 40 °C, the reaction gave the expected product 2a in 68% yield, albeit with 1
7.3 (Table 2, entry 7). When increasing the reaction temperature to 40 °C, the reaction gave the expected product 2a in 68% yield, albeit with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dr and >99% ee (Table 2, entry 8). Furthermore, on increasing the catalyst loading to 15 mol% or 20 mol%, the ee was not influenced too much but the diastereoselectivity was dramatically reduced (Table 2, entries 9 and 10). However, when the catalyst loading was decreased to 5 mol% or 2.5 mol%, the reaction displayed reduced yield and diastereoselectivity (Table 2, entries 11 and 12). Therefore, 10 mol% of dinuclear zinc catalyst ZnEt2/(S,S)-L9 and 30 mg of 4 Å MS additive were optimal reaction conditions, which were further employed to investigate the substrate scope of this intramolecular aldolization.
20 dr and >99% ee (Table 2, entry 8). Furthermore, on increasing the catalyst loading to 15 mol% or 20 mol%, the ee was not influenced too much but the diastereoselectivity was dramatically reduced (Table 2, entries 9 and 10). However, when the catalyst loading was decreased to 5 mol% or 2.5 mol%, the reaction displayed reduced yield and diastereoselectivity (Table 2, entries 11 and 12). Therefore, 10 mol% of dinuclear zinc catalyst ZnEt2/(S,S)-L9 and 30 mg of 4 Å MS additive were optimal reaction conditions, which were further employed to investigate the substrate scope of this intramolecular aldolization.
| Entrya | Solvent | Cat. (mol%) | Temp. (°C) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1a (0.1 mmol), (S,S)-L9 (10 mol%), ZnEt2 (20 mol%) and 30 mg of 4 Å MS in solvents (1 mL) at room temperature (30 °C).b Isolated yield.c Determined by 1H NMR analysis.d Determined by chiral HPLC analysis (Chiralpak AD-H). | ||||||
| 1 | Toluene | 10 | rt | 88 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
94 |
| 2 | o-Xylene | 10 | rt | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
97 |
| 3 | Et2O | 10 | rt | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
97 |
| 4 | m-Xylene | 10 | rt | 56 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
87 |
| 5 | THF | 10 | rt | 56 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
79 |
| 6 | CH2Cl2 | 10 | rt | 42 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
87 |
| 7 | o-Xylene | 10 | 20 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.3 7.3 |
91 |
| 8 | o-Xylene | 10 | 40 | 68 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
>99 |
| 9 | o-Xylene | 20 | rt | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8 4.8 |
95 |
| 10 | o-Xylene | 15 | rt | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6 5.6 |
97 |
| 11 | o-Xylene | 5 | rt | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1 6.1 |
98 |
| 12 | o-Xylene | 2.5 | rt | <10 | — | — |
The dehydration of the aldol product was also investigated in the presence of some common Brønsted acids, such as HCl, HCOOH, trifluoroacetic acid (TFA), para-toluenesulfonic acid (PTSA) and trifluoromethanesulfonic acid (TfOH). As shown in Table 3, 98% yields were respectively obtained in the presence of 10 equivalents of HCl, PTSA or TfOH (Table 3, entries 1, 4 and 5). However, the dehydration did not take place with HCOOH, and the performance of TFA was proven to be inferior to that of the above mentioned Brønsted acids (Table 3, entries 2 and 3). Finally, hydrogen chloride was selected as an optimal dehydrating regent.
| Entrya | Brønsted acid (10 equiv.) | Yieldb (%) | eec (%) |
|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 2a (0.03 mmol), and Brønsted acids (0.3 mmol), in CH2Cl2 (1 mL) for 12 hours at room temperature.b Isolated yield.c Determined by chiral HPLC analysis (Chiralpak AD-H).d 3 h. | |||
| 1 | HCl | 98 | 93 |
| 2 | HCOOH | No reaction | — |
| 3 | TFA | 68 | 93 |
| 4 | PTSA | 98 | 93 |
| 5 | TfOH | 98 | 93 |
| 6d | HCl | 50 | 93 |
Having established the optimized reaction conditions, we then explored the substrate scope and limitation of the present intramolecular aldol reaction. We found that some aldol products were susceptible to dehydration, generating the corresponding α,β-unsaturated ketone compounds. Thus, it became very difficult to separate them through column chromatography on silica gel. Fortunately, it was found that aldol products 2b–e could be isolated as the pure compounds, and their catalytic results are summarized in Table 4. Substrates 1b–e, bearing electron-donating substituents (–Me, –OMe) on the oxindole backbone, led to their aldol products 2b–e in 56–81% yields with varying levels of diastereoselectivities (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3–1
3–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and enantioselectivities (82–95%) (Table 4, entries 1–4).
20) and enantioselectivities (82–95%) (Table 4, entries 1–4).
| Entrya | Ra | Product | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1 (0.1 mmol), (S,S)-L9 (10 mol%), ZnEt2 (20 mol%) and 30 mg of 4 Å MS in o-xylene (1 mL) at 40 °C.b Isolated yield.c Determined by 1H NMR analysis.d Determined by chiral HPLC analysis (Chiralpak AD-H). | |||||
| 1 | 5-CH3 | 2b | 56 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
95 |
| 2 | 5-OCH3 | 2c | 59 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
82 |
| 3 | 5,7-2CH3 | 2d | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
93 |
| 4 | 7-Me | 2e | 81 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
86 |
Due to the difficulty of isolation of aldol products and dehydration products, the sequential aldol condensation reaction was also investigated. After the aldol reaction was judged to be completed as monitored by TLC stains, ten equivalents of hydrogen chloride were introduced into the reaction mixture. The catalytic results are summarized in Table 5. For substrates 1b–d and 1n bearing –Me or –OMe groups, the reactions proceeded smoothly to provide the desired dehydration products 3b–d and 3n in 64–79% yields with 85–92% ee, respectively (Table 5, entries 1–3 and 13). Halogen atoms such as fluorine, chlorine and bromine on different positions of the oxindole backbones obviously exerted a negative influence on the reaction, which generated the corresponding products 3e–m in 45–70% yields with 63–88% ee (Table 5, entries 4–12). Also, N–Ph and N–Bn protected diketones 1o and 1p were investigated for this sequential aldol condensation reaction, which afforded corresponding products 3o and 3p in 63% and 60% yields with 95% and 89% ee, respectively (Scheme 2). Simultaneously, single crystals of 2a were obtained from ethyl acetate and n-hexane, and the configuration of chiral stereo centers of tertiary alcohol and spiro quaternary carbon was unambiguously determined by X-ray diffraction analysis with Cu Kα radiation (λ = 1.54178 Å) (Fig. 1).
| Entrya | R | Product | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1 (0.1 mmol), (S,S)-L9 (10 mol%), ZnEt2 (20 mol%) and 30 mg of 4 Å MS in o-xylene (1 mL) at 40 °C for 12 hours. Then HCl aq. (1.0 mmol) was added in CH2Cl2 (1 mL) for another 12 hours at room temperature.b Isolated yield.c Determined by chiral HPLC analysis (Chiralpak AD-H or Chiralcel OJ-H). | ||||
| 1 | 5-CH3 | 3b | 71 | 87 |
| 2 | 5-OCH3 | 3c | 64 | 85 |
| 3 | 5,7-2CH3 | 3d | 78 | 92 |
| 4 | 5-F | 3e | 63 | 71 |
| 5 | 5-Cl | 3f | 70 | 80 |
| 6 | 5-Br | 3g | 58 | 63 |
| 7 | 4-Cl | 3h | 45 | 82 |
| 8 | 4-Br | 3i | 53 | 88 |
| 9 | 6-Br | 3j | 68 | 73 |
| 10 | 6-Cl | 3k | 55 | 75 |
| 11 | 7-Cl | 3l | 68 | 75 |
| 12 | 7-F | 3m | 55 | 82 |
| 13 | 7-Me | 3n | 79 | 86 |
 | ||
| Fig. 1 X-ray crystal structure of product 2a.19 | ||
In conclusion, a series of diketone compounds based on oxindole backbones have been designed and employed for intramolecular desymmetrizing aldol or aldol condensation reactions, which were catalyzed by a Trost dinuclear zinc catalyst. The corresponding reactions proceeded smoothly to provide spiro[cyclohexanone-oxindole] compounds in reasonable yields with moderate to good enantioselectivities.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21272166, 21572150), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (13KJA150004), the Program for New Century Excellent Talents in University (NCET-12-0743), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Scientific and Technologic Infrastructure of Suzhou (SZS201207).References
- (a) Enolates, Organocatalysis, Biocatalysis and Natural Product Synthesis, Modern Aldol Reactions, ed. R. Mahrwald, Wiley-VCH, Weinheim, Germany, 2004, vol. 1 Search PubMed; (b) L. M. Geary and P. G. Hultin, Tetrahedron: Asymmetry, 2009, 20, 131–173 CrossRef CAS; (c) B. Schetter and R. Mahrwald, Angew. Chem., Int. Ed., 2006, 45, 7506–7525 CrossRef CAS PubMed; (d) M. A. B. Ferreira, L. C. Dias, I. A. Leonarczyk, E. C. Polo and E. C. de Lucca, Curr. Org. Synth., 2015, 12, 547–564 CrossRef CAS.
- (a) W. D. Fessner, Enzyme-catalyzed Aldol Reactions, in Modern Aldol Reactions, ed. R. Mahrwald, Wiley-VCH, Berlin, 2004, vol. 1, ch. 5, pp. 201–272 Search PubMed; (b) P. Clapes, Aldol reactions, in Science of Synthesis, Biocatalysis in Organic Synthesis, ed. K. Faber, W.-D. Fessner and N. J. Turner, 2015, vol. 2, pp. 31–42 Search PubMed.
- (a) E. M. Carreira, Recent advances in asymmetric aldol addition reactions, in Catalytic Asymmetric Synthesis, ed. I. Ojima, 2nd edn, 2000, pp. 513–541 Search PubMed; (b) R. Mahrwald, Metal Catalysis, Modern Aldol Reactions, Wiley-VCH, Weinheim, Germany, 2004, vol. 2 Search PubMed; (c) T. Tsubugo, Y. Yamashita and S. Kobayashi, Top. Organomet. Chem., 2013, 45, 243–270 CrossRef CAS; (d) C. Palomo, M. Oiarbide and J. M. Garcia, Chem. Soc. Rev., 2004, 33, 65–75 RSC; (e) B. M. Trost and C. S. Brindle, Chem. Soc. Rev., 2010, 39, 1600–1632 RSC; (f) S. G. Nelson, Tetrahedron: Asymmetry, 1998, 9, 357–389 CrossRef CAS; (g) T. Zhang, Y. Zhang, Z. Li, Z. Song, H. Liu and J. Tao, Chin. J. Chem., 2013, 31, 247–255 CrossRef CAS; (h) A. Serra-Pont, I. Alfonso, C. Jimeno and J. Solà, Chem. Commun., 2015, 51, 17386–17389 RSC; (i) T. Harada and A. Abiko, The aldol reaction: transition metal enolate, in Comprehensive Organic Synthesis, ed. P. Knochel and G. A. Molander, 2nd edn, 2014, vol. 2, pp. 451–511 Search PubMed.
- (a) B. List, Amine-catalyzed Aldol Reactions, in Modern Aldol Reactions, ed. R. Mahrwald, Wiley-VCH, Berlin, 2004, vol. 1, ch. 4, pp. 161–200 Search PubMed; (b) X.-W. Wang, Y. Wang and J. Jia, Enamine Catalysis of Intramolecular Aldol Reactions, in Asymmetric Organocatalysis 1, Lewis Base and Acid Catalysts, Science of Synthesis, ed. B. List, Georg Thieme Verlag KG, Stuttgart & New York, 2012, pp. 1–33 Search PubMed; (c) S.-M. Yliniemelä-Sipari, A. Piisola and P. M. Pihko, Enamine Catalysis of Intermolecular Aldol Reactions, in Asymmetric Organocatalysis 1, Lewis Base and Acid Catalysts, Science of Synthesis, ed. B. List, Georg Thieme Verlag KG, Stuttgart & New York, 2012, pp. 35–72 Search PubMed; (d) N. Mase and Y. Hayashi, The aldol reaction: organocatalysis approach, in Comprehensive Organic Synthesis, ed. P. Knochel and G. A. Molander, 2nd edn, 2014, vol. 2, pp. 273–339 Search PubMed.
- (a) B. M. Trost and C. S. Brindle, Chem. Soc. Rev., 2010, 39, 1600–1632 RSC; (b) T. D. Machajewski and C.-H. Wong, Angew. Chem., Int. Ed., 2000, 39, 1352–1375 CrossRef CAS; (c) N. Kumagai and M. Shibasaki, Zinc polymetallic asymmetric catalysis, Multimetallic Catalysts in Organic Synthesis, ed. M. Shibasaki and Y. Yamamoto, 2004, pp. 53–76 Search PubMed; (d) A. P. Thankachan, S. Asha, K. S. Sindhu and G. Anilkumar, RSC Adv., 2015, 5, 62179–62193 RSC.
- (a) B. M. Trost and H. Ito, J. Am. Chem. Soc., 2000, 122, 12003–12004 CrossRef CAS; (b) B. M. Trost, H. Ito and E. R. Silcoff, J. Am. Chem. Soc., 2001, 123, 3367–3368 CrossRef CAS PubMed; (c) B. M. Trost, D. J. Michaelis and M. I. Truica, Org. Lett., 2013, 15, 4516–4519 CrossRef CAS PubMed; (d) G. Li, X. Wang, W. Zhao, L. Lu, G. Liu and M. Wang, Huaxue Jinzhan, 2012, 24, 348–360 CAS; (e) B. M. Trost and M. J. Bartlett, Acc. Chem. Res., 2015, 48, 688–701 CrossRef CAS PubMed.
- (a) C.-H. Wong and G. M. Whitesides, Enzymes in Synthetic Organic Chemistry, Tetrahedron Organic Chemistry Series, ed. J. E. Baldwin and P. D. Magnus, Elsevier Science & Technology, Oxford, 1994, vol. 12 Search PubMed; (b) M. T. Reetz, B. Brunner, T. Schneider, F. Schultz, C. M. Clouthier and M. M. Kayser, Angew. Chem., Int. Ed., 2004, 43, 4075–4078 CrossRef CAS PubMed.
- General reviews and book chapters: (a) E. García-Urdiales, I. Alfonso and V. Gotor, Chem. Rev., 2005, 105, 313–354 CrossRef PubMed; (b) M. C. Willis, J. Chem. Soc., Perkin Trans. 1, 1999, 13, 1765–1784 RSC; (c) A. Studer and F. Schleth, Synlett, 2005, 20, 3033–3041 CrossRef; (d) T. Rovis, Recent Advances in Catalytic Asymmetric Desymmetrization Reactions, in New Frontiers in Asymmetric Catalysis, ed. K. Mikami and M. Lautens, J. Wiley & Sons, Inc., 2007, pp. 275–309 Search PubMed; (e) I. Atodiresei, I. Schiffers and C. Bolm, Chem. Rev., 2007, 107, 5683–5712 CrossRef CAS PubMed; (f) M. D. Díaz-de-Villegas, J. A. Gálvaz, P. Etayo, R. Badorrey and M. P. López-Ram-de-Víu, Chem.–Eur. J., 2012, 18, 13920–13935 CrossRef PubMed. Selected representative examples: (g) B. M. Trost and T. Mino, J. Am. Chem. Soc., 2003, 125, 2410–2411 CrossRef CAS PubMed; (h) K. Mori, T. Katoh, T. Suzuki, T. Noji, M. Yamanaka and T. Akiyama, Angew. Chem., Int. Ed., 2009, 48, 9652–9654 CrossRef CAS PubMed; (i) Q. Gu, Z.-Q. Rong, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2010, 132, 4056–4057 CrossRef CAS PubMed; (j) C. Kanta De and D. Seidel, J. Am. Chem. Soc., 2011, 133, 14538–14541 CrossRef PubMed; (k) S. Müller, M. J. Webber and B. List, J. Am. Chem. Soc., 2011, 133, 18534–18537 CrossRef PubMed; (l) J. Ou, W.-T. Wong and P. Chiu, Org. Biomol. Chem., 2012, 10, 5971–5978 RSC; (m) L. Yao, K. Liu, H.-Y. Tao, G.-F. Qiu, X. Zhou and C.-J. Wang, Chem. Commun., 2013, 49, 6078–6080 RSC; (n) M. Wilking, C. Mueck-Lichtenfeld, C. G. Daniliuc and U. Hennecke, J. Am. Chem. Soc., 2013, 135, 8133–8136 CrossRef CAS PubMed; (o) Z.-Q. Rong, H.-J. Pan, H.-L. Yan and Y. Zhao, Org. Lett., 2014, 16, 208–211 CrossRef CAS PubMed; (p) N. Di Iorio, P. Righi, A. Mazzanti, M. Mancinelli, A. Ciogli and G. Bencivenni, J. Am. Chem. Soc., 2014, 136, 10250–10253 CrossRef CAS PubMed; (q) S.-S. Meng, Y. Liang, K.-S. Gao, L. Zou, X.-B. Lin, H. Yang, K. N. Houk and W.-H. Zheng, J. Am. Chem. Soc., 2014, 136, 12249–12252 CrossRef CAS PubMed; (r) J. Jiang, L. He, S.-W. Luo, L.-F. Cun and L.-Z. Gong, Chem. Commun., 2007, 7, 736–738 RSC; (s) H. Wu, W. Zi, G. Li, H. Lu and F. D. Toste, Angew. Chem., Int. Ed., 2015, 54, 8529–8532 CrossRef CAS PubMed; (t) A. R. Burns, A. G. E. Madec, D. W. Low, I. D. Roy and H. W. Lam, Chem. Sci., 2015, 6, 3550–3555 RSC; (u) K. Liu, H.-L. Teng, L. Yao, H.-Y. Tao and C.-J. Wang, Org. Lett., 2013, 15, 2250–2253 CrossRef CAS PubMed.
- (a) C. Agami and H. Sevestre, J. Chem. Soc., Chem. Commun., 1984, 21, 1385–1386 RSC; (b) C. Agami, N. Platzer and H. Sevestre, Bull. Soc. Chim. Fr., 1987, 2, 358–360 Search PubMed.
- B. List, R. A. Lerner and C. F. Barbas III, Org. Lett., 1999, 1, 59–61 CrossRef CAS PubMed.
- J. Zhou, V. Wakchaure, P. Kraft and B. List, Angew. Chem., Int. Ed., 2008, 47, 7656–7658 CrossRef CAS PubMed.
- (a) S. Sakai, N. Aimi, K. Yamaguchi, H. Ohhira, K. Hori and J. Haginiwa, Tetrahedron Lett., 1975, 16, 715–718 CrossRef; (b) A. Jossang, P. Jossang, H. A. Hadi, T. Sevenet and B. Bodo, J. Org. Chem., 1991, 56, 6527–6530 CrossRef CAS; (c) N. Anderton, P. A. Cockrum, S. M. Colegate, J. A. Edgar, K. Flower, I. Vit and R. I. Willing, Phytochemistry, 1998, 48, 437–439 CrossRef CAS; (d) G. Lakshmaiah, T. Kawabata, M. Shang and K. Fuji, J. Org. Chem., 1999, 64, 1699–1704 CrossRef CAS PubMed; (e) G. Cravotto, G. B. Giovenzana, T. Pilati, M. Sisti and G. Palmisano, J. Org. Chem., 2001, 66, 8447–8453 CrossRef CAS PubMed; (f) J. A. Murphy, R. Tripoli, T. A. Khan and U. W. Mali, Org. Lett., 2005, 7, 3287–3289 CrossRef CAS PubMed; (g) B. M. Trost and M. K. Brennan, Org. Lett., 2006, 8, 2027–2030 CrossRef CAS PubMed; (h) B. Yu, D.-Q. Yu and H.-M. Liu, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2964 CrossRef CAS PubMed; (b) R. Rios, Chem. Soc. Rev., 2012, 41, 1060–1074 RSC; (c) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104–6155 CrossRef CAS PubMed; (d) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC; (e) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023–1052 CrossRef CAS; (f) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS; (g) M. M. M. Santos, Tetrahedron, 2014, 70, 9735–9757 CrossRef CAS; (h) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743–762 CrossRef CAS; For selected publications, see: (i) M. Sannigrahi, Tetrahedron, 1999, 55, 9007–9071 CrossRef CAS; (j) A. Madin, C. J. O'Donnell, T. Oh, D. W. Old, L. E. Overman and M. J. Sharp, J. Am. Chem. Soc., 2005, 127, 18054–18065 CrossRef CAS PubMed; (k) B. M. Trost, N. Cramer and S. M. Silverman, J. Am. Chem. Soc., 2007, 129, 12396–12397 CrossRef CAS PubMed; (l) D. Hojo, K. Noguchi, M. Hirano and K. Tanaka, Angew. Chem., Int. Ed., 2008, 47, 5820–5822 CrossRef CAS PubMed; (m) R. Shintani, S.-Y. Hayashi, M. Murakami, M. Takeda and T. Hayashi, Org. Lett., 2009, 11, 3754–3756 CrossRef CAS PubMed; (n) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418–11419 CrossRef CAS PubMed; (o) T.-L. Liu, Z.-Y. Xue, H.-Y. Tao and C.-J. Wang, Org. Biomol. Chem., 2011, 9, 1980–1986 RSC; (p) Q.-F. Wu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 1680–1683 CrossRef CAS PubMed; (q) S. Xu, Z. Wang, Y. Li, X. Zhang, H. Wang and K. Ding, Chem.–Eur. J., 2010, 16, 3021–3035 CrossRef CAS PubMed; (r) S. Xu, Z. Wang, X. Zhang, X. Zhang and K. Ding, Angew. Chem., Int. Ed., 2008, 47, 2840–2843 CrossRef CAS PubMed; (s) Y. M. Wang, H. H Zhang, C. Li, T. Fan and F. Shi, Chem. Commun., 2016, 52, 1804–1807 RSC; (t) J. H. Li, T. F. Feng and D. M. Du, J. Org. Chem., 2015, 80, 11369–11377 CrossRef CAS PubMed; (u) F. Shi, H. H. Zhang, X. X. Sun, J. Liang, T. Fan and S. J. Tu, Chem.–Eur. J., 2015, 21, 3465–3471 CrossRef CAS PubMed; (v) W. Tan, X. Li, Y. X. Gong, M. D. Ge and F. Shi, Chem. Commun., 2014, 50, 15901–15904 RSC.
- (a) K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS PubMed; (b) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS; (c) M. M. C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein and S. L. Schreiber, J. Am. Chem. Soc., 2004, 126, 16077–16086 CrossRef CAS PubMed; (d) G. C. Bignan, K. Battista, P. J. Connolly, M. J. Orsini, J. Liu, S. A. Middleton and A. B. Reitz, Bioorg. Med. Chem. Lett., 2005, 15, 5022–5026 CrossRef CAS PubMed; (e) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (f) S. Ma, X. Han, S. Krishnan, S. C. Virgil and B. M. Stoltz, Angew. Chem., Int. Ed., 2009, 48, 8037–8041 CrossRef CAS PubMed; (g) S. Kotha, A. C. Deb, K. Lahiri and E. Manivannan, Synthesis, 2009, 2009, 165–193 CrossRef; (h) V. V. Vintonyak, K. Warburg, H. Kruse, S. Grimme, K. Hübel, D. Rauh and H. Waldmann, Angew. Chem., Int. Ed., 2010, 49, 5902–5905 CrossRef CAS PubMed.
- G. Bencivenni, L.-Y. Wu, A. Mazzanti, B. Giannichi, F. Pesciaioli, M.-P. Song, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 7200–7203 CrossRef CAS PubMed.
- Q. Wei and L.-Z. Gong, Org. Lett., 2010, 12, 1008–1011 CrossRef CAS PubMed.
- L.-L. Wang, L. Peng, J.-F. Bai, L.-N. Jia, X.-Y. Luo, Q.-C. Huang, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2011, 47, 5593–5595 RSC.
- (a) Y.-B. Lan, H. Zhao, Z.-M. Liu, G.-G. Liu, J.-C. Tao and X.-W. Wang, Org. Lett., 2011, 13, 4866–4869 CrossRef CAS PubMed; (b) B. Wu, J. Chen, M.-Q. Li, J.-X. Zhang, X.-P. Xu, S.-J. Ji and X.-W. Wang, Eur. J. Org. Chem., 2012, 7, 1318–1327 CrossRef; (c) X.-F. Huang, Z.-M. Liu, Z.-C. Geng, S.-Y. Zhang, Y. Wang and X.-W. Wang, Org. Biomol. Chem., 2012, 10, 8794–8799 RSC.
- CCDC 1011578 contains the supplementary crystallographic data for this paper†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1011578. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra02296k |
| This journal is © The Royal Society of Chemistry 2016 |