A new concept for electroless nickel plating: aluminium as reducing agent
Abstract
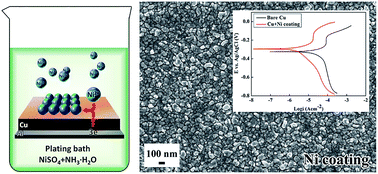
A facile electroless plating strategy to obtain nickel coatings on copper substrates was designed to simplify the plating baths and procedure. The plating baths contained only nickel sulfate and ammonia. The aluminium connected to the copper substrates served as the electron source for nickel deposition. The nickel coatings obtained via this approach were tested and proved to possess excellent anticorrosion behavior.

 Please wait while we load your content...
Please wait while we load your content...