CdIn2S4 quantum dots: novel solvent-free synthesis, characterization and enhancement of dye-sensitized solar cells performance
Abstract
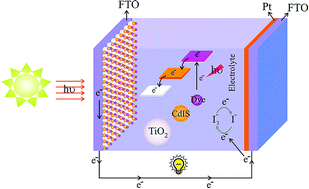
Herein, CdIn2S4 (CdIS) quantum dots were synthesized via a solid-state thermal decomposition approach for the first time. [Cd(en)2]SO4, In(NO3)3·5H2O and thioacetamide were used as the cadmium, indium and sulfur sources. The as-synthesized products were characterized extensively by techniques such as XRD, EDS, SEM, TEM, AFM, FTIR and DRS. The effect of the cadmium source type, temperature and reaction atmosphere on the morphology and purity of the final products were studied. The results showed that choosing the appropriate temperature and cadmium source has a significant influence on CdIS–QDs synthesis. Moreover, the as-prepared CdIS–QDs were utilized as a barrier layer in dye sensitized solar cells (DSSCs) for the first time. The short-circuit photocurrent density (Jsc), open-circuit voltage (Voc), fill factor (FF) and efficiency of the solar cell were studied. The power conversion efficiency of the DSSCs using a CdIS–QDs barrier layer was 8.17%, which is 32.2% higher than the cell without a novel barrier layer (6.18%).

 Please wait while we load your content...
Please wait while we load your content...