Immobilization of horseradish peroxidase enzymes on hydrous-titanium and application for phenol removal
Abstract
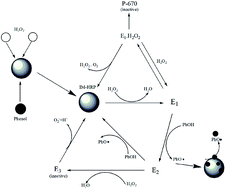
In this work, hydrous titanium was utilized to immobilize horseradish peroxidase (HRP) in order to improve its stability and adaptability under different water qualities using a biomimetic titanification process. The catalytic performance of immobilized HRP for phenol removal from aqueous solution was evaluated. The physicochemical properties of immobilized HRP (IM-HRP) were characterized by means of scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FT-IR). The effects of the reaction conditions (H2O2 and IM-HRP dosages and initial phenol concentration) on phenol removal were investigated. Enzymatic activity analysis indicated that the immobilization process has no adverse effects on the catalytic performance of HRP, and a slight increase in the enzymatic catalytic kinetic constant (km) of IM-HRP was observed compared to its free enzymes. It was found that at a synthetic temperature of 37 °C ± 3 °C, IM-HRP exhibited excellent phenol removal of over 90% after reaction for 15 min. In addition, the enzyme activity of IM-HRP was very stable over a wide range of reaction conditions (e.g., temperatures from 20 °C to 90 °C and pH from 3.0 to 11.0). Therefore, HRP enzymes loaded on hydrous titanium are very effective at improving the stability of enzymes and can alleviate the adverse effects of various environmental and water conditions on contaminant removal.

 Please wait while we load your content...
Please wait while we load your content...