Nitrogen doped holey carbon nano-sheets as anodes in sodium ion battery
Sridhar Vadahanambia,
Ho-Hwan Chunb,
Kwang Hyo Jungb and
Hyun Park*a
aGlobal Core Research Centre for Ships and Offshore Plants, Pusan National University, Busan 609-735, Republic of Korea. E-mail: hyunpark@pusan.ac.kr
bDepartment of Naval Architecture and Ocean Engineering, Pusan National University, Busan 609-735, Republic of Korea
First published on 1st April 2016
Abstract
Though the insertion mechanism of lithium and sodium ions in carbon nano-structures is similar, anodes used in sodium ion batteries face many practical difficulties due to the fact that the sodium ion is 55% larger than its lithium counterpart. One way of accommodating the spatial requirements of the large sodium ion is by increasing the ‘active surface area’ of anodes in sodium-ion batteries. In this manuscript, we developed a microwave based solvothermal technique for synthesis of nitrogen doped, holey-carbon nano sheets (hCNS) by using eco-friendly ionic liquids and potassium ethoxide. Morphological studies by SEM and TEM showed ‘in-plane’ spherical nano-pores well distributed through hCNS, whereas XPS studies showed that the nitrogen doping exists predominantly as pyridinic groups. When applied as electrodes in sodium-ion batteries, our 3D holey carbon nano-sheets show high specific capacitance of 268 mA h g−1, good rate capability and 93% of initial capacitance retention even after 200 cycles. The presence of chemically active nano-holes on the carbon nano-sheet surface increases the surface area, acts as anchoring points for sodium ions, and favours fast diffusion of the electrolyte.
1. Introduction
Secondary rechargeable batteries have become an integral part of our lives, with lithium-ion batteries (LIBs) being the most important energy storage devices and are widely used in cell phones, laptops, tablets, hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs), and full electric vehicles (EVs). However, lithium is a limited source with concentrations of 20 to 70 ppm on land and 0.14 to 0.25 ppm in sea waters, and there is the distinct possibility that in the near foreseeable future the world might run out of lithium supplies due to wide spread utilization. Though there are some efforts on recycling of lithium-ion batteries, but the general consensus is to explore “beyond lithium” based technologies for energy storage. Sodium ion batteries (SIBs) are fast emerging as alternatives to LIB due to comparatively lower cost of sodium to lithium and secondly and most importantly, due to the fact that sodium is the 4th most abundant element in the earth's crust and is widely distributed across all continents unlike lithium whose production is dominated by ‘ABC countries’ (Argentina and Australia, Bolivia and Brazil, Chile and Canada).Though there have been immense strides in development of cathode materials for SIBs, less attention has been focused on anode materials. Bare sodium metal was first investigated as anode in SIB, but practical difficulties arising from low melting point of sodium (98 °C) and consequent safety hazards have prompted researchers to look for alternatives. Theoretically, metals like germanium,1 tin,2 antimony3 act as good anodes due to their capability to form NaGe, NaxSn and Na3Sb but the problems of huge volume expansion due to insertion of sodium rules out practical applicability. Since the pioneering efforts by Komaba4,5 and Dahn6,7 groups and owing to the similar chemistry of sodium and lithium insertion in carbon-based electrode materials, researchers have investigated carbonaceous materials such as graphene,8 carbon nanotubes,9 hard carbons,10 hetero-cycle doped carbon nano assemblies,11 biochars12,13 i.e., carbons synthesized from biomaterials as anode materials in SIB. Of these graphene is worth special mention due to its unique combination of chemical and physical properties, excellent conductivity and relatively high surface area. But chemically synthesized graphene from graphite precursor suffers from poor conductivity and low ‘active surface area’ due to its tendency to restack and this re-aggregation of graphene sheets is a major hurdle in realizing its full potential. In order to avoid this, insertion of spacers like metal nano particles,14 polymers,15 carbon nanotubes,16 carbon nano-cups17 etc. have been reported. Yet another strategy to minimize restacking is disrupting the van der Waals forces of attraction by inserting holes or inducing defects through the graphene surface. These ‘in-plane’ defects in transforms graphene sheets to what is known as ‘holey-graphene’.
Hierarchical porous three dimensional carbon structures derived from a variety of precursors have been successfully synthesized and utilized as super capacitor electrode materials, and are reported to show high capacitive performance and excellent rate capability.18–22 Recent reports have shown that graphene with holes distributed along the surface can be advantageous in applications such as anode materials for lithium ion batteries and as super-capacitors due to the ease of diffusion of electrolyte through the pores in graphene. But till date, the synthesis of holey-graphene carried out in multiple steps and can be broadly classified as chemical23 and physical methods.24 In chemical method, graphene is synthesized by Hummer's method in the first step, subsequently they are decorated with metal nano-particles, and holes are drilled on the graphene surface either under external energy such as heat or by catalytic oxidation and finally the metal nano particles is etched out with repeated washing with acid. On the other hand, there are some reports on synthesis of ‘nano-mesh graphene’ by using a porous catalyst substrate in chemical vapor deposition (CVD). But in both these methods, the process is multi-stage and involves costly metals like gold or silver. So, there's a need for a simple and eco-friendly synthesis of holey graphene.
In this manuscript, we report a one-pot synthesis of holey carbon nano-sheets (hCNS) by simple microwave carbonization of ionic liquids in a soft-template. Solvothermal synthesis of graphene like carbon nano-sheets from alkali ethoxide25 offers an easier and efficient way when compared to the widely used ‘top-down (from graphite)’ and ‘bottom up (by CVD)’ synthesis. Our newly developed technique to synthesize holey carbon nano-sheets doesn't involve expensive metal etchants like gold, copper or silver and can be easily scaled up to gram scale synthesis. The suitability of our hCNS as anode materials in sodium ion batteries is also reported.
2. Experimental
2.1. Material and synthesis
The ionic liquid, 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF4) and potassium ethoxide were purchased from Merck and Aldrich respectively and were used as received. Microwave irradiation was carried out in a domestic microwave oven (Daewoo, Korea) at 700 W for 300 s. SEM micrographs were recorded using a Nova NanoSEM 230 FEI at 5 kV in gentle-beam mode without any metal coating. TEM micrographs were recorded on a JEM-3011HR microscope operated at 200 kV using a holey-carbon-coated copper grid. For both TEM and SEM studies, a drop of very dilute dispersion after settling was placed on respective substrates and dried at ambient conditions. Raman spectra were recorded using LabRAM HR UV/vis/NIR Horiba Jobin-Yvon, France at 514 nm and high resolution chemical analysis was carried out by X-ray photoelectron spectroscopy (Sigma Probe Thermo VG spectrometer using Mg Kα X-ray sources). The free ware XPSPEAK (version 4.1) was used to curve fit XPS data with a mixed Gaussian–Lorentzian shape. Electrochemical tests were conducted using CR2032 coin-type test cells assembled in argon-filled glove box. The composition of working electrodes was 80 wt% active materials (hCNS), 10 wt% ketjen black, and 10 wt% carboxy methyl cellulose binder mixed in absolute ethanol, pasted on Ni foam, and then dried at 100 °C for 12 h in a vacuum before use. Sodium foil was the counter electrode and Celgard 2400 membrane was the separator. The electrolyte was 1 M NaClO4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume of ethylene carbonate (EC) and diethyl carbonate (DEC) with 10 wt% of fluoroethylene carbonate (FEC). The working electrodes were composed of 0.5 mg of active material and a sodium foil separated by a micro-porous Celgard 2400 membrane. Galvanostatic charge–discharge cycling tests were performed using an WBCS 3000, Won-A-Tech, Korea battery testing system in the voltage range between 0.001–3 V. Post-mortem SEM analysis of electrodes was carried out after removing hCNS electrodes from the cells followed by careful washing with ethylene carbonate and drying under vacuum at 80 °C for 24 h.
1 volume of ethylene carbonate (EC) and diethyl carbonate (DEC) with 10 wt% of fluoroethylene carbonate (FEC). The working electrodes were composed of 0.5 mg of active material and a sodium foil separated by a micro-porous Celgard 2400 membrane. Galvanostatic charge–discharge cycling tests were performed using an WBCS 3000, Won-A-Tech, Korea battery testing system in the voltage range between 0.001–3 V. Post-mortem SEM analysis of electrodes was carried out after removing hCNS electrodes from the cells followed by careful washing with ethylene carbonate and drying under vacuum at 80 °C for 24 h.
3. Results and discussion
Our one-pot microwave method for synthesis of holey carbon nano-sheets is simple and utilizes two ingredients: potassium ethoxide (Sigma-Aldrich) and an ionic liquid (EMIM-BF4, Merck) and were taken in ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in acetonitrile solvent and ultra-sonicated for 60 min. Subsequently, the mixture was subjected to microwave irradiation at 700 W for about 5 min to yield a fluffy black powder. The resultant powder was washed repeatedly with 0.5 M hydrochloric acid solution and deionized water to remove the potassium salts. Subsequently, the obtained product was annealed at 700 °C for 60 min in nitrogen atmosphere to yield holey carbon nano-sheets.
1 in acetonitrile solvent and ultra-sonicated for 60 min. Subsequently, the mixture was subjected to microwave irradiation at 700 W for about 5 min to yield a fluffy black powder. The resultant powder was washed repeatedly with 0.5 M hydrochloric acid solution and deionized water to remove the potassium salts. Subsequently, the obtained product was annealed at 700 °C for 60 min in nitrogen atmosphere to yield holey carbon nano-sheets.
Ionic liquids due to their intrinsic non-volatile nature can act as rich source of carbons and are very stable until the onset of decomposition process. Based on this theory, carbonization of poly(ionic liquids) in the presence of different metallic salts have yielded an array of graphitic nano-structures such as graphene nano-sheets,26 nano tubes,27 nano-cups28 and nano-bubbles.29 In our synthesis technique, when the ionic liquid and potassium ethoxide are dissolved in acetonitrile solvent, the ionic liquid and potassium ethoxide disassociates to BF4−/imidazolium and potassium hydroxide/ethanol, respectively. Under microwave irradiation, ethanol act as ignition point and pyrolyses the imidazolium moiety of ionic liquid, resulting in sheet like graphitic frameworks. KOH, the by-product of thermal disassociation of potassium ethoxide is known to be a good physical and chemical activator of carbons and yields carbons with well defined micro-pore volume by the following chemical reactions.24
| 6KOH + 2C → 2K + 3H2 + 2K2CO3 | (1) |
| 2K2CO3 → K2O + CO2 | (2) |
| K2CO3 + 2C → 2K + 3CO | (3) |
| C + K2O → 2K + CO | (4) |
The three main steps during KOH activation of graphitic carbon obtained by carbonization of ionic liquid are: (a) chemical etching of graphitic framework by the redox reactions between the potassium compounds (K2CO3, K2O) generates in-plane porous holey graphitic network (b) evolution of CO2 and CO from the decomposition of potassium compounds contributes to physical activation, resulting in meso-pores and finally, (c) the metallic potassium intercalates into the graphitic lattices resulting in formation of KC8 which when washed with water causes expansion of graphene layers.
The SEM morphology at low and high magnification of the holey carbon nano-sheets sheets obtained by our technique is shown in Fig. 1(a). SEM micrographs shows well dispersed and uniformly distributed in-plane holes along the surface of the carbon nano-sheet. More detailed information regarding the size of the holes can be clearly observed from the TEM micrographs at low and high magnifications, presented in Fig. 1(b) and (c) respectively. From the TEM image, we can observe that irrespective of the size, all holes are spherical in shape with a bimodal distribution in size, the majority of which are in the range of 10–15 nm and minor portion falling in 100 nm range. The N2-adsorption isotherm experiments to further study the pore-size distribution and are plotted in Fig. 1(d). The N2-adsorption isotherms exhibited the combined characteristics of type I/II, with a surface area of 486 m2 g−1 and a total pore volume of 0.78 cm3 g−1. The initial region of the isotherms experienced a sharper rise at low P/P0, indicating the presence of micro-pores, and the hysteresis loop in the P/P0 range of ≈0.4–0.7 is indicative of meso-porosity. Pore-size distribution data plotted as insert in Fig. 2(d) shows that the majority of pores are below 20 nm which can be attributed to the in-plane holes on the carbon nano-sheet, a minor portion in greater than 100 nm range arising from interlayer separation and these observed values are well in agreement with the SEM and TEM results. This unique, bimodal, porous structure makes our holey-carbon nano-sheets an ideal material for applications involving transportation of electrolytes such as super-capacitor electrodes, anode materials in lithium ion batteries and in ionic polymer actuators. TEM images and BET adsorption experiments show that our developed technique results in uniform in-plane holes in carbon nano-sheets when compared to traditional metal induced oxidative etching technique for synthesis of holey-graphene.
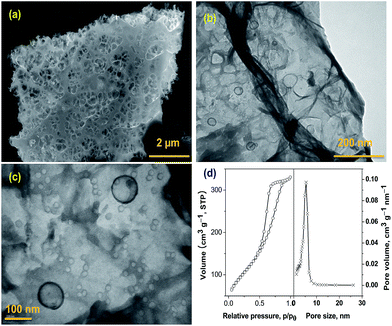 | ||
| Fig. 1 SEM (a) and TEM micrograph at low (b) and high magnification (c) and BET surface area of holey carbon nano-sheets (d). Insert in figure (d) shows the pore size distribution. | ||
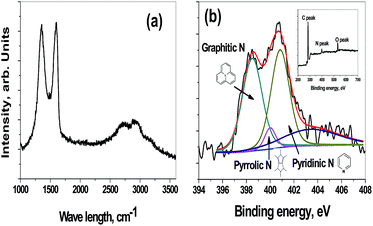 | ||
| Fig. 2 Raman spectra of holey-graphene nano sheets (a) deconvoluted N1s spectra (b) and insert in figure (b) is XPS survey scan of holey-graphene. | ||
Raman spectroscopy is a power characterization technique for studying the structure of graphitic materials. The Raman spectrum recorded for holey carbon nano-sheet (Fig. 2(a)) showed the four peaks that correspond to the G and D peaks at 1602 and 1352 cm−1, respectively and a split G′ band at 2727 and 2901 cm−1. The ratio of intensities of the D and G band (ID/IG) for graphitic materials is indicates the structural disorder of graphitic materials. The ID/IG of hCNS was 0.99 indicating a broken hexagonal symmetry of graphene. There are two reasons for this broken symmetry: first due to the in-plane porosity of carbon nano-sheets arising from well distributed nano hole as observed in TEM and secondly due to the presence of heterogeneous N-dopants. In order to check the presence of functional groups in hCNS, high resolution XPS test was carried out. The insert in Fig. 2(b) shows the survey scan indicating the presence of carbon, oxygen and nitrogen moieties in the ratios of 82.2, 11.4 and 6.4% respectively. The deconvoluted high-resolution asymmetric N1s XPS spectrum of hCNS can be was divided into three components with peaks at 398.6, 400.1 and 400.8 eV indicating that nitrogen atoms are present in three different binding states of pyridinic-N (398.7 eV), pyrrolic-N (400.1 eV), and graphitic-N (401.8 eV) consistent with the results for other N-doped carbon materials. XPS analysis shows a high percentage of pyridinic groups (∼46%) in hCNS synthesized from ionic liquids. It is known that of all the nitrogen species, the pyridinic group with the lone pair of electrons residing on the sp2 orbital, is the most electrochemically active, due to the introduction of donor states close to the Fermi level of graphene.30
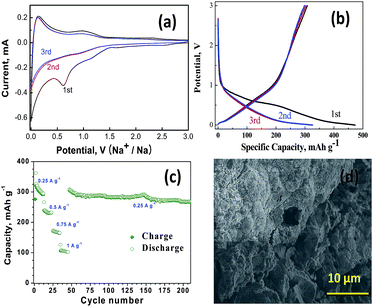
Galvanostatic cycling was carried out in the voltage range, 0.001–3 V, and at current rate of 250 mA g−1. Fig. 3(a) shows the cyclic voltammograms measured at a scan rate of 20 mV s−1 for first three cycles of our hCNS. The first cycle exhibits an irreversible reduction peak with a maximum about 0.61 V, caused by irreversible electrochemical formation of SEI layer and reduction remnant oxygen moieties in hCNS. Additionally, the reductive peak occurring at ∼0.7 V is also attributed to the formation of solid electrolyte interphase (SEI) on the surface of carbon grains. In subsequent cycles, the broad hump at 0.91 V corresponds to the decomposition of Na2O. During oxidation process, the Na-ion insertion–extraction behavior takes place in two stages with a sharp redox peak at a lower potential region (0–0.2 V) can be clearly observed, which resemble alkali-ion insertion into/extraction out of the graphite structure.6 Additionally, the broad redox peaks detected in the higher potential range of 0.67–1.0 V can be attributed to the charge transfer and Na-ion insertion in the large number of topological defects4 due to N-doping which forms a disordered carbon structure, which further enhances sodium absorption properties. The presence of nano-sized pores in hCNS effectively transports the electrolyte and storage of Na+ ions deep in the hCNS layers, which additionally contributes to the high rate performance. In the second and third cycles, the redox current peaks tend to stabilize gradually almost to the point of overlapping, which suggests that after the initial capacity decay occurring in the first cycle our hCNS electrodes shows good Na ion insertion–extraction stability.
Fig. 3(b) shows the initial discharge (sodium insertion) and charge (sodium extraction) voltage profiles hCNS anodes at 250 mA g−1 exhibiting exceptional initial discharge and charge capacity of 461 and 325 mA h g−1, respectively. The initial coulombic efficiency of 70.4% can be calculated which is primarily due to the irreversible capacity loss occurring in the formation of solid electrolyte interface (SEI). The cycling profile during first discharge showed three distinct sloping plateaus, the first occurring in the region at ∼1.5–1.25 V attributed to the Na+ insertion between the hCNS layers,7 the second plateau (∼1.25 to 1 V) to the formation of SEI film on the graphenic domains4 and the third plateau (1 to ∼0.25 V) is due to insertion of Na+ in the local disorders5 caused by nitrogen doping. In the second discharge curve, the first plateau (∼1.5–1.25 V) is almost non-existent and is replaced with a rapidly sloping curve originating at ∼1.25 V due to the domination of Na+ insertion in the graphenic structure,10 however a high discharge capacity of 298 mA h g−1 is maintained. The loss of capacity is associated with the formation of SEI in the first cycle due to trapping of Na-ions at nano-holes and formation of sodiation products (NaxCy). Recent theoretical studies by Datta et al.31 has shown that sodiation in defect rich graphene can lead to the formation of Na8C26 and Na6C32 for divalency (DV) and Stone–Wales (SW) type of defects. In our case, the defects though spherical in nature and much more bigger than both DV and SW type of defects possible leading to formation of complex NaxCy type of moieties. Consequently anodes based on our hCNS shows high discharge capacity of 268 mA h g−1 even after 200 cycles (Fig. 3(c)). Post-mortem microscopic analysis of electrodes after 200 cycles shown in Fig. 3(d) shows sodium moieties and SEI are well adhered on the flake like hCNS surface.
4. Conclusions
We developed a simple procedure for synthesis of holey graphene from ionic liquid as carbon precursor and a soft template of potassium ethoxide has been developed. Morphological studies of synthesized holey graphene by SEM and TEM techniques showed well distributed spherical holes on in-plane graphene surface with size predominantly in the range of 10–20 nm. When applied as anode in sodium ion batteries, the synthesized nanostructure exhibited high sodium storage capacity of 268 mA h g−1 even after 200 cycles emphasizing the presence in-plane nano-holes in the carbon nano sheets increase the electrolyte-accessible surface area, whereas the defects generated by substitution doping of nitrogen provide good anchoring points for sodium ion retention.Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grant of the Korea government (MSIP) through GCRC-SOP (No. 2011-0030013).References
- P. R. Abel, Y.-M. Lin, T. de Souza, C.-Y. Chou, A. Gupta, J. B. Goodenough, G. S. Hwang, A. Heller and C. B. Mullins, J. Phys. Chem. C, 2013, 117, 18885 CAS.
- M. K. Datta, R. Epur, P. Saha, K. Kadakia, S. K. Park and P. N. Kumta, J. Power Sources, 2013, 225, 316 CrossRef CAS.
- Y. Zhu, X. Han, Y. Xu, Y. Liu, S. Zheng, K. Xu, L. Hu and C. Wang, ACS Nano, 2013, 7, 6378 CrossRef CAS PubMed.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859 CrossRef CAS.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636 CrossRef CAS PubMed.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271 CrossRef CAS.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2001, 148, A803 CrossRef CAS.
- B. L. Ellis and L. F. Nazar, Curr. Opin. Solid State Mater. Sci., 2012, 16, 168 CrossRef CAS.
- X.-F. Luo, C.-H. Yang, Y.-Y. Peng, N.-W. Pu, M.-D. Ger, C.-T. Hsieh and J.-K. Chang, J. Mater. Chem. A, 2012, 3, 10320 RSC.
- A. Ponrouch, A. R. Goni and M. R. Palacin, Electrochem. Commun., 2013, 27, 85 CrossRef CAS.
- J. Xu, M. Wang, N. P. Wickramaratne, M. Jaroniec, S. Dou and L. Dai, Adv. Mater., 2015, 27, 2042 CrossRef CAS PubMed.
- J. Ding, H. Wang, Z. Li, K. Cui, D. Karpuzov, X. Tan, A. Kohandehghan and D. Mitlin, Energy Environ. Sci., 2015, 8, 941 CAS.
- J. Ding, H. Wang, Z. Li, A. Kohandehghan, K. Cui, Z. Xu, B. Zahiri, X. Tan, E. M. Lotfabad, B. C. Olsen and D. Mitlin, ACS Nano, 2013, 7, 11004 CrossRef CAS PubMed.
- S. Bai and X. Shen, RSC Adv., 2012, 2, 64 RSC.
- H. Gomez, M. K. Ram, F. Alvi, P. Villalba, E. Stefanakos and A. Kumar, J. Power Sources, 2011, 196, 4102 CrossRef CAS.
- V. Sridhar, I. Lee, H.-H. Chun and H. Park, RSC Adv., 2015, 5, 68270 RSC.
- V. Sridhar, I. Lee, H. S. Yoon, H. H. Chun and H. Park, Carbon, 2013, 61, 633 CrossRef CAS.
- L. L. Zhang, Y. Gu and X. S. Zhao, J. Mater. Chem. A, 2013, 1, 9395 CAS.
- C. Lei, N. Amini, F. Markoulidis, P. Wilson, S. Tennison and C. Lekakou, J. Mater. Chem. A, 2014, 2, 570 Search PubMed.
- T. C. Chou, C. H. Huang, R. A. Doong and C. C. Hu, J. Mater. Chem. A, 2013, 1, 6037 Search PubMed.
- X. Wang, C.-G. Liu, D. Neff, P. F. Fulvio, R. T. Mayes, A. Zhamu, Q. Fang, G. Chen, H. M. Meyer, B. Z. Jang and S. Dai, J. Mater. Chem. A, 2013, 1, 7920 CAS.
- B. Xu, H. Duan, M. Chu, G. Cao and Y. Yang, J. Mater. Chem. A, 2013, 1, 4565 CAS.
- Z. Jiang, B. Pei and A. Manthiram, J. Mater. Chem. A, 2013, 1, 7775 CAS.
- G. Ning, Z. Fan, G. Wang, J. Gao, W. Qian and F. Wei, Chem. Commun., 2011, 47, 5976 RSC.
- M. Choucair, P. Thordarson and J. A. Stride, Nat. Nanotechnol., 2008, 4, 30 CrossRef PubMed.
- J. Yuan, C. Giordano and M. Antonietti, Chem. Mater., 2010, 22, 5003 CrossRef CAS.
- V. Sridhar, H. J. Kim, J. H. Jung, S. Park and I. K. Oh, ACS Nano, 2012, 6, 10562 CAS.
- S. Soll, T. P. Fellinger, X. wang, Q. Zhao, M. Antionetti and J. Yuan, Small, 2013, 24, 4135 CrossRef PubMed.
- V. Sridhar, D. Gangaraju, H.-H. Chun and H. Park, ACS Appl. Mater. Interfaces, 2013, 5, 12323 CAS.
- L. S. Panchakarla, K. S. Subrahmanyam, S. K. Saha, A. Govindaraj, H. R. Krishnamurthy, U. V. Waghmare and C. N. R. Rao, Adv. Mater., 2009, 21, 4726 CAS.
- D. Datta, J. Li and V. B. Shenoy, ACS Appl. Mater. Interfaces, 2014, 6, 1788 CAS.
| This journal is © The Royal Society of Chemistry 2016 |