Graphene oxide based coatings on nitinol for biomedical implant applications: effectively promote mammalian cell growth but kill bacteria†
Changhong Zhao‡
ab,
Santosh Pandit‡c,
Yifeng Fubd,
Ivan Mijakovicc,
Aldo Jesorkae and
Johan Liu*ab
aSMIT Center, School of Automation and Mechanical Engineering and Institute of Nanomicro-Energy, Shanghai University, 20 Chengzhong Rd., Shanghai 201800, China. E-mail: jliu@chalmers.se
bElectronics Materials and Systems Laboratory, Department of Microtechnology and Nanoscience, Chalmers University of Technology, Kemivägen 9, SE-412 96, Gothenburg, Sweden
cSystems and Synthetic Biology Division, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden
dSHT Smart High Tech AB, Aschebergsgatan 46, SE-411 33, Gothenburg, Sweden
eDepartment of Chemistry and Chemical Engineering, Physical Chemistry, Chalmers University of Technology, Gothenburg, Sweden
First published on 11th April 2016
Abstract
An important clinical challenge is the development of implant surfaces which have good integration with the surrounding tissues and simultaneously inhibit bacterial colonization thus preventing infection. Recently, graphene oxide (GO) a derivative of graphene, has gained considerable attention in the biomedical field owing to its biocompatibility, surface functionalizability and promising antimicrobial activity. In this study gelatin-functionalized graphene oxide (GOGel) was synthesized by a simple one step modification where GO and GOGel were used to develop surface coatings on nitinol substrates. Mouse osteoblastic cell (MC3T3-E1) functions including cell attachment, proliferation and differentiation were investigated on GO-based coatings. The results indicated that MC3T3-E1 cell functions were significantly enhanced on both GO coated nitinol (GO@NiTi) and GOGel coated nitinol (GOGel@NiTi) compared with the control nitinol without coating (NiTi). Especially, the GOGel@NiTi surface exhibited the best performance for cell adhesion, proliferation and differentiation. Additionally the antimicrobial property of GO-based coatings against E. coli was studied with the evaluation of colony forming units (CFU) counting, live/dead fluorescent staining and scanning electron microscope (SEM). We found that the growth of E. coli was inhibited on GOGel@NiTi and particularly on GO@NiTi. SEM images revealed that the cell membrane of bacteria lost their integrity and live/dead fluorescent images confirmed the low live/dead ratio of E. coli after incubation on GOGel@NiTi and GO@NiTi. We conclude that GO-based coatings on NiTi combine the antimicrobial activity and improved biocompatibility and therefore present a remarkable potential in biomedical implant applications.
Introduction
Medical metallic implants such as stainless steel and titanium alloy have been widely used in orthopedic, dental and cardiovascular applications to replace a damaged biological structure in a patient's body. However, because of their biological inertness these metallic biomaterials result in lack of cell adhesion, proliferation and poor integration at the tissue–implant interface after implantation.1 Hence, surface modifications are required to improve the biocompatibility and bioactivity of such implant devices. On the other hand, implant-related infections remain among the leading reason for the failure of implant devices leading to higher economic and social associated cost.2 To prevent such infections one approach is a surface modification by coating or adding antimicrobials on implants to reduce bacterial colonization. Ideally, implant surfaces are expected to enhance the growth of living cells and simultaneously inhibit bacteria. Previously numerous materials including carbon, bioactive ceramics, hydroxyapatite (HA), and many polymers have been tested as implant coatings.3 These materials however did not meet clinical requirements for implant coatings due to a number of respective disadvantages.Since its discovery graphene has attracted considerable attention worldwide due to its exceptional electronic, thermal and mechanical properties.4–6 This allotropic type of carbon has opened new possibilities for developing novel multifunctional materials. Graphene oxide (GO) is a derivative of graphene which is modified by a high density of oxygen functional groups on the graphene sheet.7 GO exhibits excellent aqueous processability, amphiphilicity and surface functionalizability, and shows remarkable promises in biomedical applications such as bioimaging,8 biosensors,8,9 drug delivery,10,11 and scaffolds.12 Furthermore, a few studies have demonstrated the strong antimicrobial properties of GO against bacteria like Escherichia coli, Staphylococcus aureus, Bacillus subtilis, and even fungal conidia.13–17 The antimicrobial properties of GO offer a good opportunity to develop antimicrobial surface coatings on biomedical implants.
Even though the research regarding biomedical applications of graphene-based nanomaterials is expanding rapidly, relatively little is known about their influence on biological systems or intrinsic toxicity.18 Some studies on in vitro toxicity of graphene nanomaterials have demonstrated the compatibility of A549 cells,13 NIH3T3 cells,19 and embryonic cells.20 Particularly GO has been shown to enhance growth of L-929 cells,21 osteoblasts22 and kidney cells.20 Yet, there are multiple conflicting reports suggesting that GO exhibits toxicity, by damaging the cell membrane and ultimately leading to apoptosis.23,24 Similar controversy exists regarding the effect of GO on bacteria. For the same bacterial strain, E. coli, conflicting reports describe GO as being bactericidal13,17 and as supporting bacterial growth.20,25 These discrepancies may be due to differences in the surface states of the graphene-based nanomaterials tested, the degree of interaction of the tested nanomaterials with the cells during the assay or the sensitivity of the cell lines used.23 In general, the effect of graphene-based materials on biological systems is still not clear. It is challenging to understand the cell–graphene interaction due to the complexity of cellular responses and the intrinsic properties of graphene materials.
The objective of this study is to evaluate both the cytocompatibility and antimicrobial properties of two different oxygen-functionalized graphene derivatives, GO and gelatin functionalized graphene oxide (GOGel) as implant coatings. Gelatin, as its cell-binding properties have been previously demonstrated,26 was used to functionalize GO. GO and GOGel were coated on nitinol (NiTi) substrates which are widely used as biomedical implant materials. The structural and morphological characterization of the GO-based coatings was performed and as a coating state on NiTi, a detailed study of the mouse osteoblastic cell (MC3T3-E1) functions in terms of the cell spreading, growth and differentiation was provided. Additionally, we have examined the antimicrobial activities of the GO-based coatings against E. coli by using colony forming units (CFU) counting, live/dead fluorescent staining and scanning electron microscope (SEM). So far little is known about cell and bacterial responses to GO-based coatings on medical metallic implants. Our study may thus not only provide a potential biomaterial for implant coating but also contribute to a better understanding of the intrinsic toxicity of engineered graphene-based nanomaterials.
Experimental section
Preparation of GO-based coating on NiTi substrates
GO was prepared from natural graphite powder by modified Hummers method.27 Briefly, concentrated H2SO4 was used to produce graphite oxide (GtO) by oxidation of graphite powder (Sigma-Aldrich). As-produced GtO was washed thoroughly with deionized water to remove chemical residues and then re-dispersed in deionized water. GO was obtained by bath-sonicating (BRANSON Digital Sonifier 450) GtO dispersion for 3 h.For the preparation of gelatin-functionalized graphene oxide (GOGel), a 5 mg ml−1 aqueous gelatin solution was first prepared by dissolving 100 mg of gelatin in 20 ml deionized water with stirring 30 min at 60 °C. Then the GO aqueous dispersion (1 mg ml−1) was dropped into the gelatin solution. After magnetically stirring for 12 h at 60 °C, the resulting mixture dispersion was centrifuged under 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed 3 times with hot water to remove unbound gelatin residues. Finally, the gelatin stabilized GO was collected and re-dispersed in water with a final concentration of 0.5 mg ml−1.
000 rpm and washed 3 times with hot water to remove unbound gelatin residues. Finally, the gelatin stabilized GO was collected and re-dispersed in water with a final concentration of 0.5 mg ml−1.
Nitinol alloy (NiTi, 50 at% Ni; 50 at% Ti) was used as the substrate for applying GO-based coating. Briefly, NiTi discs (14 mm in diameter) were pretreated by sequentially polishing with diamond polishing papers grits #400, #600, #800 and #1200. The NiTi substrates were treated with acid by immersing in 30% H2O2 solution overnight followed by washing with water and acetone, further drying by nitrogen gun. GO and GOGel coatings were obtained by dropping 0.1 ml GO or GO–Gel aqueous suspension (0.1 mg ml−1) on the NiTi surface and dried at room temperature.
Characterization
The morphology and size of GO sheet was characterized by Atomic Force Microscopy (AFM, Bruker Dimension 3100), Raman spectra of GO was obtained by Raman microscope (Horiba). We also employed Scanning Electron Microscope (SEM, Zeiss Supra 60 VP), Fourier transform infrared spectroscopy (FTIR, PerkinElmer Spectrum Two), and X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD) to characterize and analyze GO based coatings on NiTi.Protein adsorption
Fibronectin (FN) and bovine serum albumin (BSA) adsorption was performed on GO-based coatings. GO coated NiTi (GO@NiTi), GOGel coated NiTi (GOGel@NiTi) and the control (NiTi without coatings) were immersed in FN (50 μg ml−1) and BSA (100 μg ml−1) PBS solution. After incubation for 3 h, samples were rinsed with PBS and then the adsorbed proteins on coating surfaces were removed by immersing in 1% sodium dodecyl sulfate (SDS). The concentrations of proteins were measured with a MicroBCA protein assay kit (Sigma). Furthermore, FITC labelled BSA (Sigma) solution (at a concentration of 50 μg ml−1) was incubated on the coating surfaces for 3 h. Then the samples were rinsed in PBS 3 times and mounted for visualization with a confocal microscopy (ZEISS LSM 700).Mammalian cell culture
Mouse MC3T3-E1 cells (ATCC subclone 14) were cultured in α-Minimum Essential Media (α-MEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco). The cultures were incubated in an atmosphere with 5% CO2, 95% air at 37 °C. All GO@NiTi, GOGel@NiTi samples and NiTi controls were sterilized overnight through exposure to 70% ethanol prior to in vitro study.Immunofluorescence microscopy and cell morphology
Cells were seeded on all coated samples in 24-well plates with a density of 2 × 104 cells per cm2. After culturing for 24 h in the medium, cells were fixed by 4% paraformaldehyde (PFA) in PBS solution for 15 min. Cells were permeabilized in 0.2% Triton X-100 in PBS for 10 min, then blocked by 1% BSA. After a 30 min blocking, cells were stained first with mouse monoclonal anti-vinculin antibody (Sigma-Aldrich) for 1 h at 37 °C and rinsed with PBS thoroughly and then incubated with a goat-anti-mouse IgG-FITC secondary antibody (Sigma) for another 1 h at room temperature. For the actin and nucleus staining, rhodamine–phalloidin (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI, Sigma) were added in the secondary antibody solution. After extensive washing with PBS, cells were observed using confocal laser scanning microscopy (ZEISS LSM 700).The cell spreading and binding on GO-based coatings was detected with SEM. After fixation with 2.5% glutaraldehyde, cells were dehydrated with gradient ethanol solution (30%, 40%, 50%, 70%, 95%, and 100%) followed by a supercritical point drying. Finally, cells were sputter-coated with a gold–palladium layer and observed with SEM (Zeiss Supra 60 VP) at an accelerating voltage of 5 kV.
Cell attachment, viability and proliferation
MC3T3-E1 cells were seeded in 24-well plates which contained GO@NiTi, GOGel@NiTi, and NiTi control with a density of 1 × 104 cells per cm2. After 4 h, 1, 2 and 4 days of culture cell numbers were determined using cell counting kit-8 (Sigma-Aldrich) following the instruction from the manufacturer. For direct visualization of living cells on the coatings, 1 × 104 cells were seeded on each surface, after 2 days of incubation, cells were stained by fluorescein diacetate (FDA, Sigma) and observed using a confocal laser scanning microscopy.Cell differentiation
After 14 days of culture in differentiation media containing α-MEM medium supplemented with 50 mg ml−1 ascorbic acid (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich), cells were rinsed with PBS twice, trypsinised, removed from the coated surface and then re-suspended in α-MEM medium. The cell solution was centrifuged at 1000 rpm for 5 min. After removal of the supernatant, the cell pellet was suspended in 1 ml of 0.2% Nonidet P-40 solution and sonicated in ice-water bath for 2 min. The alkaline phosphatase (ALP) activity and calcium content were determined using a fluorescence-based ALP detection kit (Sigma) and a QuantiChrom calcium assay kit (BioAssay) following the manufacturer's instruction, respectively.Antimicrobial ability evaluation
E. coli was employed to evaluate the antimicrobial performance of the GO-based coatings. After sterilization in 70% ethanol aqueous solution, bacterial cell suspension at a concentration of 107 CFU ml−1 was introduced onto the coated surface to a density of 60 μl cm−2. After 24 h of incubation at 37 °C, the dissociated bacterial solution was collected and spread onto a standard agar culture medium and left to grow for 24 h at 37 °C. Colony counting method was applied to evaluate the viability of E. coli on GO-based coatings. All the tests were performed two times in triplicate.Furthermore, live/dead fluorescent staining was performed to show the viability of bacteria on the samples, bacteria at a concentration of 107 CFU ml−1 were inoculated on the sample surfaces. After 24 h of incubation, the culture medium was removed and the samples were rinsed with physiological saline, stained by using a LIVE/DEAD BacLight™ Bacterial Viability Kit (L13152, Molecular Probes) for 15 min in dark, and then observed by fluorescence microscopy (Olympus GX71).
The morphological changes of bacterial cells were further investigated using SEM. Bacterial cell suspensions were diluted to obtain cell samples containing 1 × 107 CFU ml−1, and introduced on the sample with a density of 60 μl cm−2. After incubation for 24 h, cells were fixed with 2.5% glutaraldehyde and then dehydrated with a series of ethanol solutions (30, 50, 75, 90, 95, and 100%) for 15 min each sequentially. The dried cells were sputter-coated with gold for SEM imaging.
Results and discussion
Characterization of GO and GO-based coatings
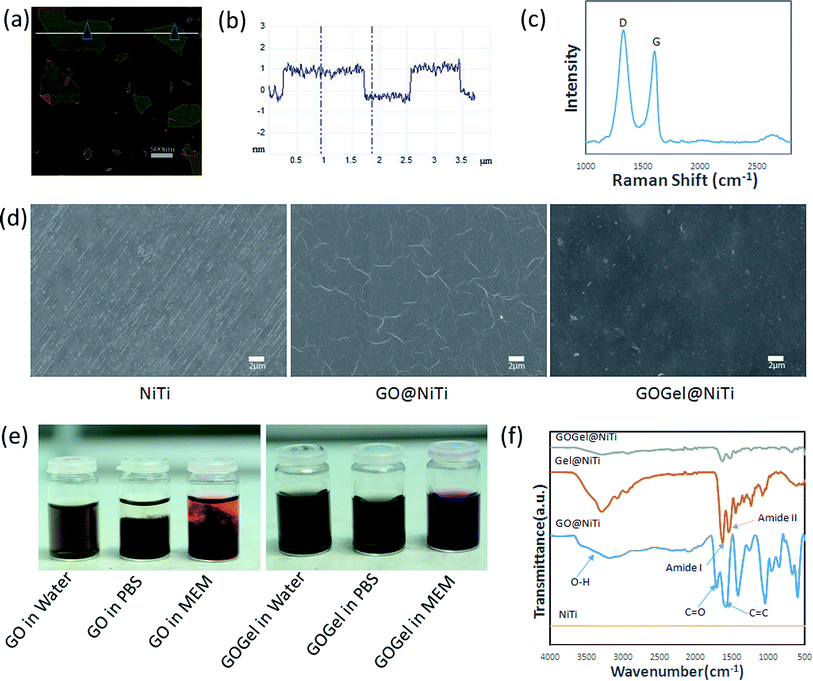
Graphene oxide nanosheets were prepared by the modified Hummers method in this study. The obtained GO was resuspended in water and diluted to obtain a GO solution with a concentration of 50 μg ml−1, then dropped on a clean silicon wafer for AFM analysis. AFM imaging revealed that the thickness of the GO sheets produced was around 1 nm (Fig. 1a and b), indicating single-layer GO sheets were prepared.28 Raman spectroscopy is a widely used tool for the characterization of carbon structures, as shown in Fig. 1c, two representative bands were observed in the Raman spectrum of GO. The G band (1590 cm−1) is due to sp2 bonding from the carbon while the D band (1350 cm−1) reflects the disorder level introduced on the sp2 hybridized hexagonal sheet of carbon by the presence of defects.29The stability of GO and gelatin functionalized GO (GOGel) in various physiological solutions were investigated by dispersing them in water, PBS, and MEM cell culture medium, respectively. As shown in Fig. 1e, after 3 days, GO at 0.2 mg ml−1 was soluble and stable in water but aggregated in solutions containing salts or proteins such as PBS and MEM cell medium. This could be attributed to screening of the electrostatic surface charge and non-specific binding of proteins on the GO sheets that broke the equilibrium state of GO in solutions.30 However, the resulting GOGel (0.2 mg ml−1) exhibited excellent stability in all biological solutions tested and no aggregation was observed after 3 days. This highly improved dissolubility might be due to the gelatin functionalization, which was further confirmed by FTIR and XPS. Fig. 1f depicts the FTIR spectrum of the GO, GOGel, and gelatin coatings on NiTi substrates. No obvious characteristic peaks were detected on pristine NiTi control, indicating that the alloy was not oxidized. For GO coating, the presence of different type of oxygen functionalities was confirmed at 3400 cm−1 (a broad peak due to O–H stretching vibrations from O–H groups), at 1710 cm−1 (stretching vibrations from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carboxyl group), and at 1418 cm−1 arising from the C–OH carboxyl group. The peak at 1576 cm−1 reflects the aromatic C
O in the carboxyl group), and at 1418 cm−1 arising from the C–OH carboxyl group. The peak at 1576 cm−1 reflects the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.31 After functionalization by gelatin, the peaks for amide-I at 1620 cm−1 and amide-II at 1500 cm−132 present in the pristine gelatin coating were also detected in GOGel coating. In addition, the IR spectrum of the GOGel indicates significant decrease of the intensity of the oxygen containing groups suggesting that GO was reduced.
C bond.31 After functionalization by gelatin, the peaks for amide-I at 1620 cm−1 and amide-II at 1500 cm−132 present in the pristine gelatin coating were also detected in GOGel coating. In addition, the IR spectrum of the GOGel indicates significant decrease of the intensity of the oxygen containing groups suggesting that GO was reduced.
The chemical composition changes of the NiTi substrates before and after coating were analyzed by XPS. Fig. S1 (ESI†) shows the wide scan spectra of XPS for GO@NiTi, GOGel@NiTi, and the NiTi control surface. Compared to pure NiTi, C, O and N peaks were also observed but the intensity of Ti2p and Ni2P peaks decreased dramatically and almost disappeared after coated with GO and GOGel. The results indicate that a homogeneous coating was formed on the NiTi surface. After coupling with gelatin, both the carbon and nitrogen peaks increased significantly on the GOGel@NiTi surface, comparing with GO@NiTi. These changes clearly confirmed the functionalization of GO.
The morphology of GO@NiTi, GOGel@NiTi and the NiTi surface were investigated using SEM (Fig. 1d). Compared to the pristine NiTi surface, GO coating with wrinkles was clearly seen on GO@NiTi. However, the scratches on NiTi surface were not seen on both GO@NiTi and GOGel@NiTi, indicating the NiTi surface was fully covered by the coatings. Wrinkles of the GO sheets were not observed on GOGel@NiTi surface. The difference in morphology implies that the structure of GO nanosheet was changed due to the gelatin coupling.
Protein adsorption on coated surfaces
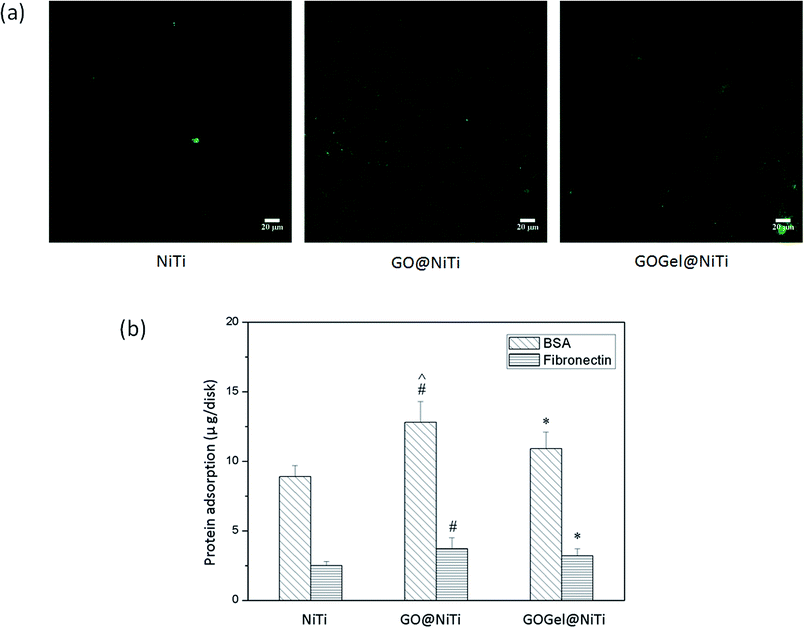
Protein adsorption is the first event when materials surfaces contact biological environment and is highly related to the biocompatibility of materials.33 Therefore, the adsorptions of fibronectin (FN) and bovine serum albumin (BSA) on the coated surfaces was examined. As shown in Fig. 2b, the BSA adsorption was determined to be 12.8 ± 1.5 μg and 10.9 ± 1.2 μg on GO@NiTi and GOGel@NiTi respectively, which were significantly higher than that on NiTi (Student's t-test, p < 0.05). As for the adsorption of FN, a similar trend was detected. For example, the amounts of adsorbed FN on GO@NiTi was 30% higher than that on NiTi, similarly, the FN adsorption on GOGel @NiTi was 25% higher than that on NiTi control. Both for BSA and FN, GO@NiTi was observed to have the strongest ability of protein adsorption. To visualize the adsorbed proteins on different surfaces, FITC labelled BSA in PBS solution was incubated with the sample surfaces for 3 h. Fig. 2a shows the fluorescence images of the BSA adsorption on GO@NiTi, GOGel@NiTi and NiTi surfaces. The morphology and nanostructure of the surface dramatically affects the protein adsorbtion to various substrates. For example, GO@NiTi surface with wrinkles exhibited higher protein adsorption than GOGel@NiTi and smooth NiTi surface. Interestingly, the high density of adsorbed protein on GO@NiTi caused a honeycomb structure with uniform pores. However, GOGel@NiTi showed a pattern of stripes after BSA adsorption, while the smooth NiTi surface formed a thin and uniform protein layer. The fluorescence intensities of the images increased in the order of NiTi, GOGel@NiTi and GO@NiTi that means the amounts of proteins adsorbed increased in the same order. And this protein adsorption trend was consistent with the quantitative measurements as shown in Fig. 2b.Protein adsorption to implant surfaces is a common but very complicated phenomenon. The interactions between proteins and surfaces are influenced by various factors, including the protein properties, surface properties and the external parameters such as temperature, pH, ionic strength, etc.34 From the surface property side, important parameters which are highly related to regulating protein adsorption include surface energy, polarity, charge, and morphology.35,36
Protein–material interactions include the redistribution of charged groups of proteins on the material surface, hydrophobic interactions between protein and the material surface, and structural rearrangements of protein molecules.37 In this study, three different surfaces were tested. Compared to NiTi surface, GO@NiTi, and GOGel@NiTi contain oxygenous groups from GO, which introduce charged and electronegative regions to the surface and enable the formation of hydrogen bonds with proteins. However, the electrostatic forces between proteins and GO are unfavorable for protein adsorption. Specifically, BSA has an isoelectric point of ∼4.7 and is negatively charged in pH 7.2 buffer.24 The oxygenous groups might induce more electrostatic repulsion to negatively charged BSA proteins. The reason for strongest adsorption on GO@NiTi surface may be a mixture contribution of hydrogen bonding, hydrophobic interaction and structural rearrangements of protein molecules. However, the structural rearrangements of proteins caused by the surface morphology should be expected to play a critical role, since the surfaces showed totally different morphologies after protein adsorption (Fig. 2a). The coated surface, both GO@NiTi and GOGel@NiTi showed improved protein adsorption compared to the NiTi control. This is probably due to the GO-based coatings affecting the protein adsorption by changing the surface nano-morphology and topography. Compared to GO@NiTi, GOGel@NiTi surface showed a different morphology without winkles and exhibited a lower protein adsorption. This also implies the important relationship between protein adsorption and surface morphology.
MC3T3-E1 cell growth on coated surfaces
Cell adhesion on the coated surfaces was investigated by immunostaining of vinculin and actin. GO@NiTi and GOGel@NiTi showed enhanced adhesion compared with the control NiTi, as indicated by immunostaining studies in Fig. 3a. Particularly, cells on the GOGel@NiTi surface exhibited greater spread with higher confluence of vinculin and actin than that on GO@NiTi and NiTi. Most cells plated on GOGel@NiTi spread out well and organized actin into stress fibers. Moderate spreading was observed on GO@NiTi, while the cells on NiTi showed a more slim shape with less spread. This finding demonstrated that the cell adhesion increased after coating. Cell morphologies after a 1 day culture on different surfaces were revealed by SEM (Fig. 3b). Cells on NiTi exhibited a poor spreading with small cell size and contacting area, whereas those on GO@NiTi and GOGel@NiTi had a better spreading with more filopodia formed around the cellular body. Consistently, GOGel@NiTi was observed to have a best cell adhesion with a complete spreading to the underlying surface.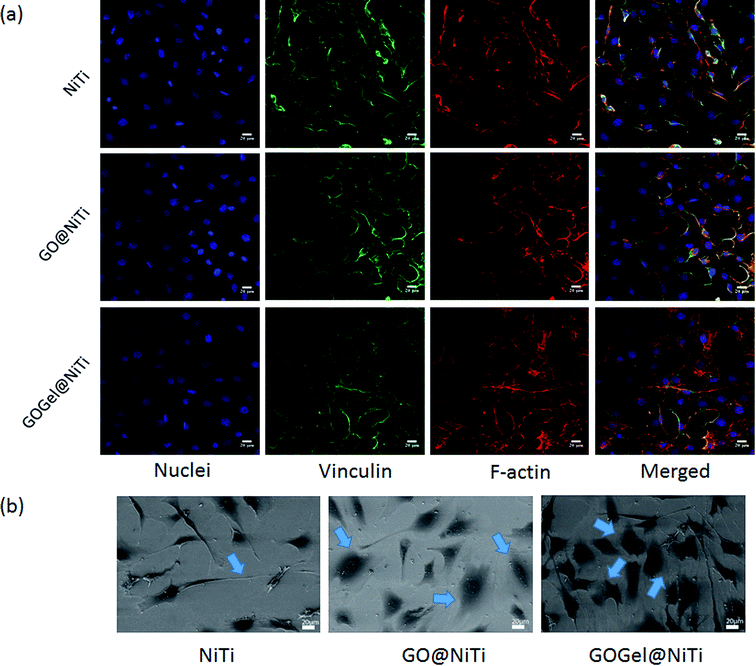 | ||
| Fig. 3 Cell adhesion and spreading on GO@NiTi, GOGel@NiTi and NiTi surfaces. (a) Immunostaining and (b) SEM images of MC3T3-E1 on various surfaces. | ||
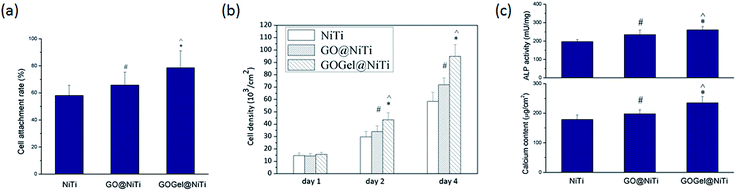
To determine the effect of GO based coatings on cell attachment, viability and proliferation, cell number and density on different coated surfaces was recorded at 3 h, day 1, 2 and 4 after seeding by the CCK-8 assay. As shown in Fig. 4a, cell attachment rate measurements revealed that more cells adhered on GOGel@NiTi in comparison to GO@NiTi and NiTi control. After a 3 h culture, more than 80% cells attached on the GOGel@NiTi, which is significantly higher than that on GO@NiTi and NiTi control. The difference was smaller but still significant for GO@NiTi in comparison to cell attachment on NiTi control surface. This result suggests that cell attachment was enhanced by GO-based surface coatings. The enhancement of the cell adhesion on such coated surface may be attributed to the improved protein adsorption and the successful immobilization of gelatin. It is known that the extracellular matrix (ECM) proteins such as laminin, fibrous collagen, and fibronectin play an important role in regulating cellular events including adhesion, proliferation and migration.38 Once cells are exposed to the material, ECM proteins selectively precipitate on material surface and provide integrin receptors for cellular recognition and cell anchorage.39 Arginine–glycine–aspartic acid (RGD) sequences, the functional structure found in ECM proteins, are able to promote cell adhesion and proliferation by interaction with integrin receptors.40 A number of ECM proteins which are rich in RGD, such as collagen, hyaluronan and fibrin have been used for surface modification.41–43 Gelatin, a partially hydrolyzed collagen, possesses the RGD sequences of collagen, making it highly effective for cell adhesion.42 In addition, ECM proteins such as fibronectin from the culture medium can be adsorbed on the sample surface and play a positive role in cell adhesion. The effective adsorption of fibronectin is likely to be the reason for the increased cell attachment on GO@NiTi. GOGel@NiTi, exhibits weaker adsorption of proteins but a higher cell adhesion than GO@NiTi. The reason for this might be a combination of protein adsorption and gelatin bonding effects, with the latter playing a major role.
Cell viability on various surfaces was evaluated by staining the living cells, as indicated in Fig. S2 (ESI†), the number of living cells increased in the order of NiTi (lowest cell viability), GO@NiTi, and GOGel@NiTi (highest cell viability). The improved cell viability on coated NiTi suggests that GO-based coatings are biocompatible. Moreover, the degree of cell proliferation on coated surfaces was investigated by cell counting.
The proliferation results (Fig. 4b) showed that cells proliferated on all surfaces as the culture time increased. No significant differences in cell proliferation were observed at day 1. However, at day 2 and 4, the coated surfaces exhibited greater cell growth than the NiTi control surface. The cell density rank was GOGel@NiTi > GO@NiTi > NiTi. The biggest differences were observed at day 4, with the cell density on GOGel@NiTi 1.5-fold higher than that on NiTi and GO@NiTi 1.2-fold higher than on NiTi. To sum up, GOGel@NiTi had the highest level of proliferation, NiTi had the weakest level of proliferation while GO@NiTi exhibited a moderate level of proliferation. Our results are consistent with previously published reports that GO film,22,25 while not exerting any cytotoxic effects on the cells, actually promotes mammalian cell attachment and proliferation. We noticed that results regarding the cytotoxicity of graphene-based nanomaterials obtained by different authors are conflicting. Particularly for GO, a few reports on in vitro toxicity of graphene nanomaterials suggest that GO materials are cytotoxic to human erythrocytes and skin fibroblasts by leading to plasma membrane damage, impairment of mitochondrial activity, induction of oxidative stress and DNA damage.23 Literature data indicate that GO could produce cytotoxicity in dose- and time-dependent means, and can enter human lung fibroblasts cytoplasm and nucleus, inducing cell floating and apoptosis at doses above 20 μg ml−1 after 24 h.44 In addition, Liao et al.45 showed that the exposure environment (i.e., whether or not GO aggregation occurs) and mode of interaction with cells effect the toxicity of GO significantly. Little is known regarding to the genotoxicity of GO, limited knowledge hypothesis that GO may reduce lifespan through influencing the functions of insulin/IGF signaling, TOR signaling, and germline signaling pathways controlled by miRNAs provided.46 It is known that, analogous to other carbon nanomaterials, physico-chemical characteristics (such as the size,47 the degree of oxidation24) of GO and the degree of interaction of the tested GO with the biological cells may play a critical role in the biological activity of this nanomaterial. However, some results have shown that GO in the form of film or coating can exhibit excellent biocompatibility with no viability inhibition of investigated cells. In this study, GO as a coating showed improved cell adhesion and proliferation without toxicity. Since we used a continuous film coating, the mechanical damage to the cell membrane induced by the sharp edges of GO sheets were presumably lowered or even avoided to some degree. Additionally, the adsorbed protein layer might have affected the GO coating-cell contact and interaction, thus enhancing cell proliferation, consistent with the protein adsorption results. For GOGel@NiTi, gelatin functionalization is expected to be the major reason for the enhancement of cell proliferation, as gelatin is a well-known cell adhesion mediator and its ability to increase cell attachment and proliferation has been previously described.48 The enhanced cell viability and proliferation on GOGel@NiTi indicates that the biocompatibility and bio-function of GO based materials can be improved by bonding functional biomolecules.
As a consequence of the enhanced cell adhesion and proliferation on coated surfaces, the cell differentiation was also promoted significantly as shown in Fig. 4c. The intracellular ALP activity from cells on GOGel@NiTi was significantly higher than that on GO@NiTi and NiTi control. GO@NiTi showed a moderate level of ALP production, 1.2-fold higher than that of the control NiTi. ALP activity is regarded as an early marker of osteoblast differentiation, the ALP activity increased in the order of NiTi, GO@NiTi, and GOGel@NiTi, indicating higher differentiation level in the same order. Osteoblast differentiation in vitro and in vivo can be characterized in three stages: (a) cell proliferation, (b) matrix maturation, and (c) matrix mineralization.49 The calcium content in osteoblasts reveals the level of matrix mineralization and osteoblast differentiation. After a 14 days culture, the calcium content ranking was GOGel@NiTi > GO@NiTi > NiTi. These results were consistent with the ALP assay. The enhanced differentiation of MC3T3-E1 cells on GO-based coatings might be attributed to the better cell adhesion and proliferation. The cell confluence occurred earlier when the proliferation was faster. Consequently, the cells on GO@NiTi, especially on GOGel@NiTi, reached confluence faster than NiTi control and subsequently the cells launched the osteogenesis earlier on GO@NiTi and especially on GOGel@NiTi. A recent study showed that the presence of gelatin improved the interaction between GO and calcium ions, and promoted the nucleation of HA during the biomimetic mineralization.26 This report also supported the notion that gelatin-functionalized GO showed improved bioactivity and a promising potential in bone tissue engineering.
Antibacterial activity of GO based coatings
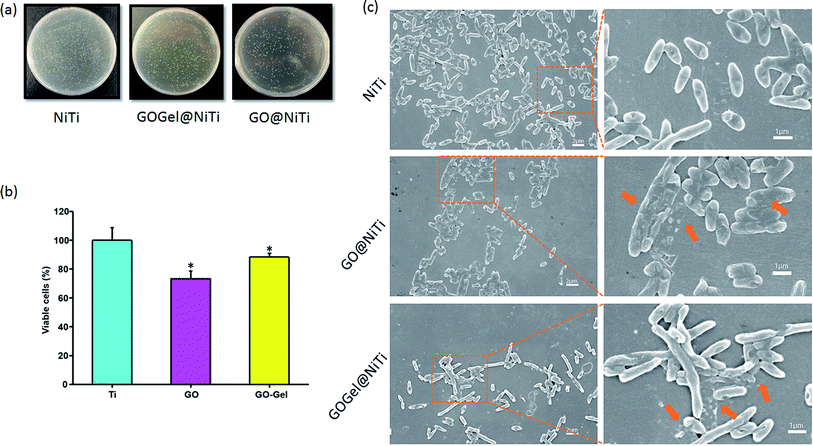
Recently, some studies have shown that graphene and graphene oxide have strong antibacterial activity towards E. coli,13,17 while being a non-specific enhancer of E. coli cellular growth in other cases.20,25 As we discussed earlier, these conflicting results may be due to differences in the surface states of the GO, the degree of interaction of GO with the cells or the sensitivity of the strains used in the assays. Here, we evaluated the antibacterial performance against E. coli of GO-based coatings on NiTi substrates by counting colony forming units (CFU), live/dead fluorescent staining and scanning electron microscopy (SEM). In order to investigate the effects of GO based coatings on the growth of E. coli, bacterial cells were introduced onto the different substrates, i.e., GOGel@NiTi, GO@NiTi, and NiTi. After 24 h of incubation, the attached bacteria were dissociated from the surfaces, re-cultivated on agar, and the colonies were counted and recorded. Fig. 5a shows the typical photographs of E. coli colonies on three types of surfaces. The number of colonies was decreased in the order of NiTi, GOGel@NiTi, and GO@NiTi. E. coli cell growth was inhibited on GOGel@NiTi, and particularly on GO@NiTi compared with the NiTi control surface. The cell viability was quantified and shown in Fig. 5b. Small but statistically significant loss of viability was observed on GO@NiTi and GOGel@NiTi surfaces.Although the underlying molecular mechanisms remain unclear, graphene- and GO-induced toxicity is thought to arise from the bacterial cell membrane damage, resulting from direct interactions between the graphene and bacteria.6 Our results confirmed the damage of the E. coli cell membranes after incubation with GO based coatings. As shown in Fig. 5c, the cells on the NiTi control surface maintained normal shape and structure. However, for coated surfaces, particularly for GO@NiTi (Fig. 5c the second line), a number of bacterial cells in the visible field at low magnification showed membrane damage and cytoplasm leakage, indicated by the orange arrow at high magnification. The damage to E. coli cells in our SEM images is consistent with previous images obtained by transmission electron microscope.13 It was reported that the graphene-induced degradation of cell membranes is caused by severe insertion and cutting and by destructive extraction of lipid molecules.50 This insertion and cutting mechanism is a likely scenario for the graphene suspension. However, as a coating, GO-bacteria contact possesses a different geometry. In graphene suspension, membrane damage induced through sheet penetration or phospholipid extraction by GO is mainly mediated by orthogonal contact with GO sheet edges.47 In contrast, for GO-based surface coatings, bacteria cells interacted with basal planes of GO mainly due to flat sheet stacking, and orthogonal cell contact with sheet edges is reduced. Therefore, the probability of GO sheets insertion into the bacterial cells or cutting the cells by sharp edges is reduced. Instead, the membrane stress on bacteria cells caused by interaction with GO-coated surfaces might be a major reason for the cell damage. GO-induced membrane stress on E. coli cells might also arise from the effects of oxidative stress, which is regarded as another important contributor for the antimicrobial activity of GO.51 The oxidation of proteins, lipids, and nucleic acid can be caused by oxidative stress in bacterial cells and ultimately leads to membrane damage and cell death.47
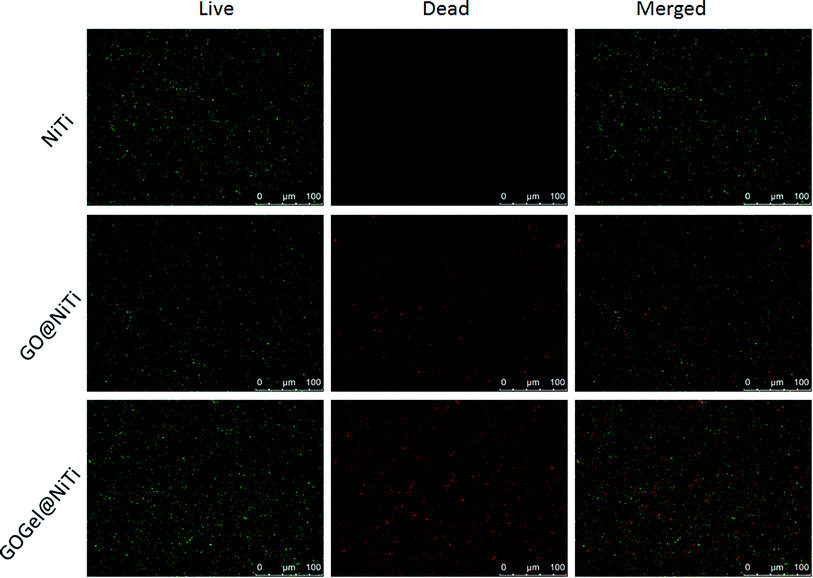
Live/dead fluorescent staining was also used to visualize and to verify the capability of the GO-based coatings to reduce the viability of bacteria on respective surfaces. The representative images from fluorescence microscopy are presented in Fig. 6. After 24 hours of bacterial growth on surfaces, most bacteria were observed viable (green) on the NiTi surface. On the contrary, the amounts of viable bacteria are evidently lower and the dead cells (red) increased dramatically on the GO@NiTi and GOGel@NiTi surfaces. Interestingly, GOGel@NiTi surface showed highest intensity both in green and red, indicating that the total number of cells attached on GOGel@NiTi is higher than the other surfaces. This improved cell adhesion might be due to the gelatin bonding. Overall, both coated surfaces showed antibacterial activities confirmed by large number of dead cells. This result is consistent with the cell viability check by CFU counting.
Conclusions
In conclusion, gelatin-functionalized graphene oxide (GOGel) was synthesized by a facile route, GO and GOGel were used to develop coating on NiTi surfaces. A detailed characterization of the GO-based coatings, including FTIR, XPS, and SEM was performed. The success of gelatin bonding was confirmed by FTIR and XPS. Both mammalian cell MC3T3-E1 and bacteria E. coli responses to the coated surfaces were studied. GO-based coatings were found to show good biocompatibility to MC3T3-E1 cells but were toxic to E. coli. MC3T3-E1 cell functions, including cell adhesion, spreading, proliferation, and differentiation, were enhanced on GO@NiTi, and especially on GOGel@NiTi in comparison to the NiTi control surface. The enhanced cell performance on GO@NiTi may be due to the higher protein adsorption than that on NiTi while the gelatin bonding should play a major role for the enhancement of cell growth on GOGel@NiTi. The strong antibacterial activities against E. coli were shown on GO@NiTi, and the damage of bacteria cell membrane caused by GO surface was clearly observed in SEM images. With the incorporation of gelatin, GOGel@NiTi showed a moderate but significant bactericidal effect. Our results indicated that GO-based coatings exhibit good biocompatibility and improve the osteoblast cell functions, while effectively killing bacteria. These findings suggest that GO-based coatings represent a promising new venue in surface modification of biomedical implants.Acknowledgements
This work was supported by the National Science Foundation, China under the contract no. 51272153 and 61574088 and carried out within the Sustainable Production Initiative and the Production Area of Advance at Chalmers. The work on bacterial toxicity was supported by the grant from the Chalmers Graphene Centre and the Chalmers Areas of Advance Nanoscience and Nanotechnology and Life Science Engineering to IM. In addition to this, the work is also supported by the Area of Advance: Production at Chalmers University of Technology. The authors acknowledge the Centre for Cellular Imaging at the Sahlgrenska Academy, University of Gothenburg for the use of imaging equipment and for the support from the staff.References
- C.-M. Xie, et al., Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties, ACS Appl. Mater. Interfaces, 2014, 6(11), 8580–8589 CAS.
- J. Schierholz and J. Beuth, Implant infections: a haven for opportunistic bacteria, J. Hosp. Infect., 2001, 49(2), 87–93 CrossRef CAS PubMed.
- M. Xuereb, J. Camilleri and N. J. Attard, Systematic review of current dental implant coating materials and novel coating techniques, Int. J. Prosthodont., 2014, 28(1), 51–59 CrossRef PubMed.
- A. K. Geim, Graphene: status and prospects, Science, 2009, 324(5934), 1530–1534 CrossRef CAS PubMed.
- C. E. N. E. R. Rao, et al., Graphene: The New Two-Dimensional Nanomaterial, Angew. Chem., Int. Ed., 2009, 48(42), 7752–7777 CrossRef CAS PubMed.
- S. Liu, et al., Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress, ACS Nano, 2011, 5(9), 6971–6980 CrossRef CAS PubMed.
- S. Park and R. S. Ruoff, Chemical methods for the production of graphenes, Nat. Nanotechnol., 2009, 4(4), 217–224 CrossRef CAS PubMed.
- D. Du, Y. Yang and Y. Lin, Graphene-based materials for biosensing and bioimaging, MRS Bull., 2012, 37(12), 1290–1296 CrossRef CAS.
- N.-F. Chiu, et al., Graphene oxide-based SPR biosensor chip for immunoassay applications, Nanoscale Res. Lett., 2014, 9(1), 1–7 CrossRef CAS PubMed.
- R. Justin and B. Chen, Characterisation and drug release performance of biodegradable chitosan–graphene oxide nanocomposites, Carbohydr. Polym., 2014, 103, 70–80 CrossRef CAS PubMed.
- K. Kostarelos and K. S. Novoselov, Exploring the interface of graphene and biology, Science, 2014, 344(6181), 261–263 CrossRef CAS PubMed.
- S. H. Ku and C. B. Park, Myoblast differentiation on graphene oxide, Biomaterials, 2013, 34(8), 2017–2023 CrossRef CAS PubMed.
- W. Hu, et al., Graphene-based antibacterial paper, ACS Nano, 2010, 4(7), 4317–4323 CrossRef CAS PubMed.
- L. Hui, et al., Availability of the basal planes of graphene oxide determines whether it is antibacterial, ACS Appl. Mater. Interfaces, 2014, 6(15), 13183–13190 CAS.
- K. V. Krishna, et al., Graphene-based nanomaterials for nanobiotechnology and biomedical applications, Nanomedicine, 2013, 8(10), 1669–1688 CrossRef CAS PubMed.
- J. Zhao, et al., Graphene Oxide-Based Antibacterial Cotton Fabrics, Adv. Healthcare Mater., 2013, 2(9), 1259–1266 CrossRef CAS PubMed.
- S. Kulshrestha, et al., A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans, Biofouling, 2014, 30(10), 1281–1294 CrossRef CAS PubMed.
- W. C. Lee, et al., Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide, ACS Nano, 2011, 5(9), 7334–7341 CrossRef CAS PubMed.
- S.-R. Ryoo, et al., Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies, ACS Nano, 2010, 4(11), 6587–6598 CrossRef CAS PubMed.
- S. Park, et al., Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite, Adv. Mater., 2010, 22(15), 1736–1740 CrossRef CAS PubMed.
- H. Chen, et al., Mechanically strong, electrically conductive, and biocompatible graphene paper, Adv. Mater., 2008, 20(18), 3557–3561 CrossRef CAS.
- S. Agarwal, et al., Interfacing live cells with nanocarbon substrates, Langmuir, 2010, 26(4), 2244–2247 CrossRef CAS PubMed.
- T. Lammel, et al., Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2, Part. Fibre Toxicol., 2013, 10(1), 27 CrossRef CAS PubMed.
- X. Shi, et al., Regulating Cellular Behavior on Few-Layer Reduced Graphene Oxide Films with Well-Controlled Reduction States, Adv. Funct. Mater., 2012, 22(4), 751–759 CrossRef CAS.
- O. N. Ruiz, et al., Graphene oxide: a nonspecific enhancer of cellular growth, ACS Nano, 2011, 5(10), 8100–8107 CrossRef CAS PubMed.
- H. Liu, et al., Gelatin functionalized graphene oxide for mineralization of hydroxyapatite: biomimetic and in vitro evaluation, Nanoscale, 2014, 6(10), 5315–5322 RSC.
- W. S. Hummers Jr and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef.
- M. J. McAllister, et al., Single sheet functionalized graphene by oxidation and thermal expansion of graphite, Chem. Mater., 2007, 19(18), 4396–4404 CrossRef CAS.
- D. R. Dreyer, et al., The chemistry of graphene oxide, Chem. Soc. Rev., 2010, 39(1), 228–240 RSC.
- B. J. Hong, et al., Successful stabilization of graphene oxide in electrolyte solutions: enhancement of biofunctionalization and cellular uptake, ACS Nano, 2011, 6(1), 63–73 CrossRef PubMed.
- M. Wojtoniszak, et al., Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide, Colloids Surf., B, 2012, 89, 79–85 CrossRef CAS PubMed.
- M. Pérez-Mateos, P. Montero and M. Gómez-Guillén, Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends, Food Hydrocolloids, 2009, 23(1), 53–61 CrossRef.
- M. Rabe, D. Verdes and S. Seeger, Understanding protein adsorption phenomena at solid surfaces, Adv. Colloid Interface Sci., 2011, 162(1), 87–106 CrossRef CAS PubMed.
- V. Hlady, J. Buijs and H. P. Jennissen, Methods for studying protein adsorption, Methods Enzymol., 1999, 309, 402 CAS.
- W. Song and H. Chen, Protein adsorption on materials surfaces with nano-topography, Chin. Sci. Bull., 2007, 52(23), 3169–3173 CrossRef CAS.
- R. Zheng, et al., Regulation of keratinocyte expression of stress proteins and antioxidants by the electrophilic nitrofatty acids 9-and 10-nitrooleic acid, Free Radicals Biol. Med., 2014, 67, 1–9 CrossRef CAS PubMed.
- F. A. Denis, et al., Protein adsorption on model surfaces with controlled nanotopography and chemistry, Langmuir, 2002, 18(3), 819–828 CrossRef CAS.
- S.-C. Wu, et al., Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds, J. Bioact. Compat. Polym., 2011, 0883911511423563 Search PubMed.
- C. Zhao, et al., Combination of positive charges and honeycomb pores to promote MC3T3-E1 cell behaviour, RSC Adv., 2015, 5(53), 42276–42286 RSC.
- R. Cancedda, et al., Tissue engineering and cell therapy of cartilage and bone, Matrix Biol., 2003, 22(1), 81–91 CrossRef CAS PubMed.
- W. Godbey and A. Atala, In vitro systems for tissue engineering, Ann. N. Y. Acad. Sci., 2002, 961(1), 10–26 CrossRef CAS PubMed.
- E. Rosellini, et al., Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering, J. Biomed. Mater. Res., Part A, 2009, 91(2), 447–453 CrossRef PubMed.
- Z. Han, et al., EDB Fibronectin Specific Peptide for Prostate Cancer Targeting, Bioconjugate Chem., 2015, 26(5), 830–838 CrossRef CAS PubMed.
- W. Gongming, et al., Microbial reduction of graphene oxide by Shewanella, Nano Res., 2011, 4(6), 563–570 CrossRef.
- K. H. Liao, et al., Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts, ACS Appl. Mater. Interfaces, 2011, 3(7), 2607–2615 CAS.
- W. Qiuli, et al., microRNAs control of in vivo toxicity from graphene oxide in Caenorhabditis elegans, Nanomedicine: Nanotechnology, Biology and Medicine, 2014, 10(7), 1401–1410 CrossRef PubMed.
- F. Perreault, et al., Antimicrobial properties of graphene oxide nanosheets: why size matters, ACS Nano, 2015, 9(7), 7226–7236 CrossRef CAS PubMed.
- M. Müller, et al., Spectroscopic and thermodynamic characterization of the adsorption of plasma proteins onto cellulosic substrates, in Macromolecular Symposia, Wiley Online Library, 1996 Search PubMed.
- G. S. Stein, J. B. Lian and T. A. Owen, Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation, FASEB J., 1990, 4(13), 3111–3123 CAS.
- Y. Tu, et al., Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets, Nat. Nanotechnol., 2013, 8(8), 594–601 CrossRef CAS PubMed.
- K. Krishnamoorthy, et al., Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation, J. Phys. Chem. C, 2012, 116(32), 17280–17287 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06026a |
| ‡ Changhong Zhao and Santosh Pandit contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |