The different effect of reduced graphene oxide and graphene oxide on the performance of chitosan by using homogenous fillers
Tiannan Zhou,
Xiaodong Qi,
Hongwei Bai and
Qiang Fu*
College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China. E-mail: qiangfu@scu.edu.cn; Fax: +86-28-8546-1759; Tel: +86-28-8546-1759
First published on 30th March 2016
Abstract
How reduced graphene oxide (rGO) and graphene oxide (GO) affect the performance of chitosan (CS) nanocomposites is discussed in this paper. A special two-step reduction method was used to prepare the CS–rGO nanocomposite films, firstly, the CS–GO nanocomposite films were prepared by the solution casting method, and secondly, the prepared CS–GO films were immersed into a reducing agent aqueous solution in which the reducing agents could diffuse onto the surface of the GO sheets and then reduce them. So this method could avoid the phenomena of aggregation, morphology change and rearrangement of the GO sheets, which would happen if they are directly reduced in the CS–GO solution with or without a surfactant. The results show that the loading of the two kinds of fillers can enhance the tensile strength of the nanocomposites, but the mechanism is different, one reason is due to the strong interfacial interaction between GO and CS, and another one may be due to the high mechanical strength of rGO and the recrystallization of the CS matrix during the reduction process. This work provides a new way to analyze the interfacial interaction between the filler and CS matrix, and also could be used in other polymer systems to find the essential mechanism of how the filler could affect on the mechanical properties of nanocomposites.
1. Introduction
Graphene, since its discovery in 2004,1 which is a single layer of carbon atoms in a closely packed honeycomb two-dimensional lattice, has attracted a large amount of attention due to its unique properties such as excellent mechanical properties,2,3 electrical conductivity,4,5 and specific surface area.6 Due to these excellent mechanical properties, graphene has opened up new pathways for developing nanocomposites materials with excellent mechanical properties.7,8The graphene based polymer nanocomposites can be prepared through the covalent or non-covalent functionalized graphene.9,10 The graphene oxide (GO) is a convenient functional graphene to prepare the well-dispersed homogeneous nanocomposites because of the large oxygen functional groups (e.g. hydroxyl, epoxide, and carbonyl groups) attached on the surface and edge. These polar functional groups of GO could improve the interfacial interaction between them and polymer matrix, such as polyvinyl alcohol,11 polyallylamine,12 polystyrene.13 The chitosan (CS) has multiple functional groups such as hydroxyl, amino groups and ether bond, these groups make it dissolve in acetic aqueous solution and have well compatibility with GO.14,15 A series of works have already been conducted on using the GO as nanofillers to improve the strengths of CS, due to its well dispersion and orientation in the chitosan matrix,15,16 but in the CS–rGO nanocomposites, the surfactant is needed to disperse the rGO sheets in CS matrix.17 In both of above-mentioned condition, the dispersion, morphology and the arrangement of rGO sheets in CS matrix are different from that of GO sheets in CS matrix, so it maybe lead the different mechanism of the effect of GO and rGO on the nanocomposites' mechanical properties.
In this paper, we discuss the essential mechanism of the effect of GO and rGO on the mechanical properties of CS matrix by preparing the homogenous CS–GO and CS–rGO films, and avoid the difference of fillers’ size, dispersion and morphology in CS matrix. This method has two steps, the first step is the preparation of CS–GO nanocomposites films; and the second step is to immerse the above-mentioned CS–GO films into reducing agents aqueous solution for several minutes to reduce the GO sheets; finally, dry them and the CS–rGO films are prepared.
2. Materials and methods
2.1. Materials
Artificial graphite power was purchased from Qingdao Black Dragon Graphite Co., Ltd. CS powder from Crab Shell, with a deacetylation degree of 85% and viscosity of 140 mPa s for the aqueous solution of 1 wt% chitosan and 1 wt% acetic acid (HAc aqueous), was bought from Zhejiang Golden-Shell Biochemical Co. Ltd. (Yuhuan, China). Potassium permanganate (KMnO4), sulfuric acid (H2SO4 98%), hydrogen peroxide (H2O2) and sodium nitrate (NaNO3), sodium hydroxide (NaOH), HAc, and Na2S2O4 were purchased from Kermel Chemical reagent plant (Tianjin, China).2.2. Preparation of GO
GO was obtained by using Hummer's method.18 Briefly, graphite (2 g) was mixed with NaNO3 (1 g) and H2SO4 (50 ml) at 0 °C, then KMnO4 (6 g) was slowly added in one hour. The reaction mixture was kept at 0 °C for 2 h. After removal of the ice-bath, the mixture was stirred at room temperature for 30 min. Distilled water (100 ml) was slowly added to the reaction, keeping the temperature below 98 °C for 3 h. The mixture was further treated with 5% H2O2 (50 ml), filtered and washed with hot water until residual salts and acids were completely removed. Finally, the GO was obtained by freeze-drying.2.3. Preparation of CS–rGO nanocomposites
CS–GO nanocomposites containing different amount of filler were prepared as follows: GO was dissolved in 20 mL of water and treated with ultrasound for 15 min at room temperature to yield a clear solution. 2 wt% CS aqueous solutions was prepared by dissolving CS (1 g) in a 1% (v/v) HAc aqueous solution, and be stirred at 200 rpm for 1 h, then filtered to remove the impurity under vacuum. A desired amount of GO suspension was gradually dropped into the CS solution, and stirred at 60 °C for 1 h. No GO aggregation was observed in a few hours after magnetic stirring. The homogeneous CS–GO solution was poured into a Teflon dish and kept at 60 °C for 15 h to cast film and then peeled it off for next reducing.The NaOH and Na2S2O4 were used to reduce GO and the detail of this reduction process is described in our previous work.19 Here, the CS–GO film was immersed in reducing agents solution (12.5 mg ml−1 of Na2S2O4 and 50 mg ml−1 of NaOH) at 60 °C for different time to yield different degree of reduction CS–rGO nanocomposites. Then those prepared nanocomposite films were rinsed with distilled water until completely remove the residual material (including reducing agents and byproduct Na2SO3 (ref. 19)). Blank sample was prepared by immersing the CS–GO films into the aqueous solution (12.5 mg ml−1 of Na2SO4 and 50 mg ml−1 of NaOH) at 60 °C for one hour. Finally, nanocomposite films were cut into specimens in the size with 60 × 10 mm by using a razor blade, and had been dried at 50 °C for 5 h under vacuum before mechanical test.
2.4. Characterizations
Microscopic morphology observations were conducted with a FEI Inspect F scanning electron microscopy (SEM) under an acceleration voltage of 5 kV.The tensile experiments were carried out on an Instron 5567 universal testing machine equipped with a 100 N load cell. Samples were tested with a crosshead speed of 5 mm min−1 with the gauge length of 20 mm. The reported values were calculated from averages over five specimens for each group of specimen.
Wide-angle X-ray diffraction (XRD) pattern of the samples were obtained with X'Pert Pro X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm) under a voltage of 40 kV and a current of 40 mA. The range of the diffraction angle (2θ) of samples was scanned from 1° to 45° with the scan speed of 0.5° min−1 at room temperature.
The resistance of films was measured with Keithley 6487 electrometer. Two-point method was used. Silver paint was applied to both ends of the sample to ensure good contact. As a result, contact resistance is negligible comparing to sample resistance. The bulk conductivity was calculated with eqn (1)
| σ = L/(RS) | (1) |
Fourier transform infrared spectra (FTIR) of the nanocomposites in transmission mode were obtained in KBr pellets using Nicolet 6700 spectrometer (Thermo Electron Corporation).
TGA (TGA Q500, TA Instruments) was performed under N2 flow, and samples were heated from room temperature to 600 °C at a rate of 10 °C min−1, and the char of them were kept for SEM observation.
3. Results and discussions
3.1. Dispersion and reduction degree of rGO
Due to the strong interaction between oxygen-functional groups on the surface of GO sheets and amino, hydroxyl groups in the unit of CS in acetic acid aqueous solution, the homogeneous dispersion of CS and GO on the molecular scale could be achieved.15 The well-dispersed CS–rGO nanocomposites films could be fabricated first by casting CS–GO solution to films and then reducing these composite films. This method could avoid the change of filler's dispersion and morphology when GO sheets are directly reduced in CS–GO solution. Fig. 1A shows homogeneous and brown CS–1 wt% GO nanocomposite film, which is flat and shiny. After being reduced, the film becomes black and opaque. Also, it is still strong and flexible, and can be bent into large angle (Fig. 1B).The residual material (including reducing agents and byproduct Na2SO3) is completely removed judged by SEM picture of fracture surface (Fig. 2A), in which the white salt crystal can't be observed, Fig. 2B shows the one in which the residual material doesn't be removed at all for the visible white salt crystal.
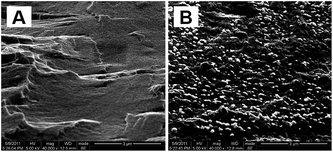 | ||
| Fig. 2 The SEM pictures of CS–rGO film fracture surface with (A) and without (B) residual materials. | ||
The cryo-fracture surfaces of films are investigated by SEM for observing the morphology of rGO in CS matrix. The fracture surface of pure CS film is smooth comparing with a layered structure for the composite film as shown in Fig. 3A.17,20 The rGO sheets (maybe single layer or multi-layer) are coated with CS matrix through the hydrogen bonding between the CS chains and residual oxygen-functional groups on the surface of rGO sheets. The morphological difference of cryo-fracture surface between CS and nanocomposites film further supports that the rGO sheets are uniformly dispersed and embedded in the CS matrix. Besides that, the reduction process would not have negative effect on the dispersion of rGO sheets in CS matrix.
The reduction degree is detected by the change of electrical conductivity; three samples with different GO contents were used to define the efficient reduced time (RT). The conductivity of the CS–0.7 wt% rGO film doesn't change no matter how long the RT is, as shown in Fig. 4, because the conductive network doesn't exist in this film, so the efficient RT can't be defined. When increasing the addition of filler, the conductivity of samples (CS–3 wt% rGO and CS–7 wt% rGO) sharply increase to 5.5 × 10−5 S m−1 and 1.7 × 10−3 S m−1 as RT reaches 15 min, further increase to 7.1 × 10−4 S m−1 and 1.6 × 10−2 S m−1 when RT reaches 1 h, and there is little change when RT reaches 5 h. So it can be concluded that the efficient RT is 1 h. The CS–rGO films bellow-mentioned were just reduced for 1 h.
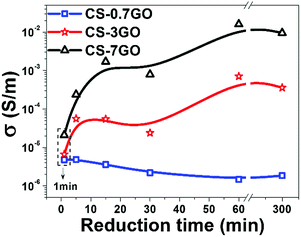 | ||
| Fig. 4 The electrical conductivity of CS–0.7 wt% GO, CS–3 wt% GO and CS–7 wt% GO films under different reduced time. | ||
3.2. Electrical conductivity
The Fig. 5 shows plots of electrical conductivity versus rGO content for CS–rGO nanocomposites, the conductivity of CS–0.5 wt% rGO is higher than the antistatic criterion (1 × 10−6 S m−1).21 The conductivity was increased by 3 orders of magnitude to 7.1 × 10−4 S m−1 for the sample of CS–3 wt% rGO and further increases to 1 × 10−1 S m−1 with the 10 wt% rGO loading. The power law is used to analyze electrical conductivity:22| σ ∝ σ0(φ − φc)ν | (2) |
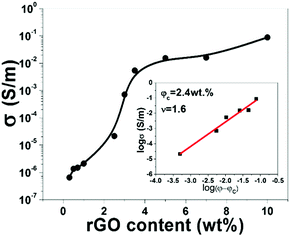 | ||
| Fig. 5 The electrical conductivity of CS–rGO films as a function of rGO content; the inset is double-logarithmic plot of volume electrical conductivity versus (φ − φc). | ||
3.3. Mechanical properties
The mechanical behaviors of CS and its nanocomposites films are investigated by mechanical test at 18 °C. The typical stress–strain curves of films are shown in Fig. 6A. Only with a little addition of GO or rGO, the tensile strength of CS matrix can be greatly improved. The tensile strength increases by 44% from 93 MPa to 137 MPa and 50% from 93 MPa to 140 MPa with the addition of 0.7 wt% rGO and 0.7 wt% GO respectively. But the addition of GO has negative effect on the elongation at break which decreases from 15% to 9% in CS–GO nanocomposites as shown in Fig. 6B. The probable factors which influence on mechanical properties are discussed in the following. | ||
| Fig. 6 (A) Stress–strain curves of CS, CS–0.7 wt% rGO and CS–0.7 wt% GO; (B) the tensile strength and elongation at break for nanocomposites as a function of filler content. | ||
Due to the homogenous of CS–GO and CS–rGO, the distribution and arrangement of filler could not affect the mechanical properties, so the crystallization and interfacial interaction are discussed to find out the reason why the difference of enhanced tensile strength exists.
XRD is used to investigate the effect of different fillers on the recrystallization of CS matrix. Fig. 7A shows the XRD patterns of CS, CS–0.7 wt% GO and CS–0.7 wt% rGO. The blank CS film shows two peaks around 2θ = 10.3 and 20.3° in the XRD spectrum. The former peak corresponds to the crystalline structure, while the broad peak around 2θ = 20.3° indicates the existence of amorphous structure.25 The XRD pattern of CS–0.7 wt% GO shows the weaker crystalline peak and sharper amorphous peak than that of blank CS (Fig. 7A), but the curve of CS–0.7 wt% rGO has stronger crystalline peak around the 10.3° than CS–0.7 wt% GO. These results indicate that the existence of GO would retard the recrystallization of CS chains during the reduction process, but the rGO doesn't.
The FTIR (Fig. 7B) is used to further investigate the interaction between the filler and matrix. The spectrum of CS shows several characteristic absorbance bands, in which the peak at 3400 cm−1 is corresponded to O–H stretching vibration, peaks at 1031 cm−1 and 1159 cm−1 are attributed to primary alcoholic group of C6–OH and the secondary alcoholic group of C3–OH, and the peak at 1637 cm−1 are assigned to carbonyl stretching vibration of acetylated amino group. The peaks of the characteristic absorption in the curve of film are similar with that of CS, which indicates the reducing agents would not react with CS. The peak at 1632 cm−1 is obvious in CS films, the characteristic of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of –NHCO–, which shifts to a lower wavenumber (1629 cm−1) at the loading of 0.7 wt% GO, but shifts to a higher wavenumber (1644 cm−1) when GO are reduced. The peak around 3432 cm−1, which corresponds to the –OH stretching vibration, shows the same variation trend with the peak the C
O stretching vibration of –NHCO–, which shifts to a lower wavenumber (1629 cm−1) at the loading of 0.7 wt% GO, but shifts to a higher wavenumber (1644 cm−1) when GO are reduced. The peak around 3432 cm−1, which corresponds to the –OH stretching vibration, shows the same variation trend with the peak the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. Due to the oxygen-functional group on the GO surface, the hydrogen bonding (C
O stretching vibration. Due to the oxygen-functional group on the GO surface, the hydrogen bonding (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N) would be formed between GO and amino groups of the CS matrix, cause the peak of the C
O⋯H–N) would be formed between GO and amino groups of the CS matrix, cause the peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration downshifting. The loss of oxygen-functional groups on the GO surface leads the decreased intensity of hydrogen bonding during the reduction process, and weakens interface interaction between the CS matrix and rGO. So the well interaction between GO and CS causes the efficient loading transfer and improve the tensile strength, but simultaneously, it would restrict the movement of CS chains and decrease the elongation of CS–GO nanocomposites.26 Combining the XRD results, the well interaction between CS matrix and GO restricts the movement of CS chains, and causes the stronger tensile strength than that of CS–rGO nanocomposites; meanwhile, the rGO has weaker interaction with CS matrix, which doesn't limit the movement of CS chains, and induces the recrystallization of CS matrix during the reduction process.
O stretching vibration downshifting. The loss of oxygen-functional groups on the GO surface leads the decreased intensity of hydrogen bonding during the reduction process, and weakens interface interaction between the CS matrix and rGO. So the well interaction between GO and CS causes the efficient loading transfer and improve the tensile strength, but simultaneously, it would restrict the movement of CS chains and decrease the elongation of CS–GO nanocomposites.26 Combining the XRD results, the well interaction between CS matrix and GO restricts the movement of CS chains, and causes the stronger tensile strength than that of CS–rGO nanocomposites; meanwhile, the rGO has weaker interaction with CS matrix, which doesn't limit the movement of CS chains, and induces the recrystallization of CS matrix during the reduction process.
It indicates that the interfacial interaction between the CS and GO is the decisive factor for improving the tensile strength of CS–GO nanocomposite; meanwhile, in the CS–rGO nanocomposites, the induced recrystallization of CS matrix during the reduction process is the decisive factor to improve the mechanical properties.
3.4. Thermal property
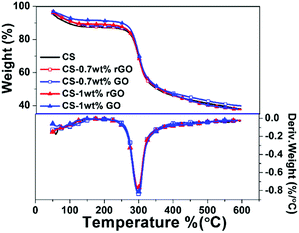
TGA is used to test the thermal property of CS and nanocomposites (Fig. 8). All samples are stored at 20 °C and the relative humidity is 30% for five mouths. Two step of non-oxidative degradation is observed under nitrogen flow. The first weight loss between 50 and 200 °C is attributed to the absorbed water in chitosan, and the second weight loss between 200 °C and 400 °C is corresponded to the degradation and deacetylation of chitosan, reported by others' research.27–29 In general, the rGO has much better thermal stability than GO, due to lose the most oxygenated functionalities groups and the recovered carbon lattice, but the addition of GO or rGO doesn't improve the thermal stability of CS nanocomposite. There are maybe two reasons, one is due to the decomposed temperature of GO (200–250 °C) lower than that of CS matrix (∼300 °C), and another one is due to the rGO sheets has the high heat conductivity, which couldn't effectively restrict the heat transfer in nanocomposites.The char of chitosan nanocomposites after TGA tests are observed by SEM (Fig. 9), results show the surface of char is flat (the first row), even be magnified by 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times (the second row), there is still no obviously difference between CS–GO and CS–rGO films. So the thermal property of films is unchanged before and after reduction.
000 times (the second row), there is still no obviously difference between CS–GO and CS–rGO films. So the thermal property of films is unchanged before and after reduction.
 | ||
| Fig. 9 SEM images of the char of chitosan nanocomposites after TGA tests: (1) CS–0.7 wt% GO; (2) CS–0.7 wt% rGO; (3) CS–1 wt% GO; (4) CS–1 wt% rGO. | ||
4. Conclusions
In this work, the effect of GO and rGO on the mechanical properties of CS nanocomposites has been discussed. By comparative study, removing the interference of the size, dispersion and arrangement of fillers in the nanocomposite, the results show that: (1) the hydrogen bonding between the CS chains and GO is the key factor to influence the mechanical properties of CS–GO nanocomposites; (2) the induced recrystallization of CS matrix during the reduction process is the key factor to influence that of the CS–rGO nanocomposite. Besides that, the loading of GO or rGO doesn't have negative influence on the thermal stability of CS matrix.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385 CrossRef CAS PubMed.
- C. Gómez-Navarro, M. Burghard and K. Kern, Nano Lett., 2008, 8, 2045 CrossRef PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS.
- S. Biswas and L. T. Drzal, ACS Appl. Mater. Interfaces, 2010, 2, 2293 CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS PubMed.
- G. Eda and M. Chhowalla, Nano Lett., 2009, 9, 814 CrossRef CAS PubMed.
- D. Chen, X. Wang, T. Liu, X. Wang and J. Li, ACS Appl. Mater. Interfaces, 2010, 2, 2005 CAS.
- H. J. Salavagione, M. A. Gómez and G. Martínez, Macromolecules, 2009, 42, 633 CrossRef.
- K. Jo, T. Lee, H. J. Choi, J. H. Park, D. J. Lee and D. W. Lee, Langmuir, 2011, 27, 2014 CrossRef CAS PubMed.
- J. Liang, Y. Huang, L. Zhang, Y. Wang, Y. Ma and T. Guo, Adv. Funct. Mater., 2009, 19, 2297 CrossRef CAS.
- S. Park, D. A. Dikin, S. T. Nguyen and R. S. Ruoff, J. Phys. Chem. C, 2009, 113, 15801 CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney and E. A. Stach, Nature, 2006, 442, 282 CrossRef CAS PubMed.
- M. Fang, J. Long, W. Zhao, L. Wang and G. Chen, Langmuir, 2010, 26, 16771 CrossRef CAS PubMed.
- X. Yang, Y. Tu, L. Li, S. Shang and X. Tao, ACS Appl. Mater. Interfaces, 2010, 2, 1707 CAS.
- Y. Pan, T. Wu, H. Bao and L. Li, Carbohydr. Polym., 2011, 83, 1908 CrossRef CAS.
- X. Wang, H. Bai, Z. Yao, A. Liu and G. Shi, J. Mater. Chem., 2010, 20, 9032 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 133 CrossRef.
- T. Zhou, F. Chen, K. Liu, H. Deng and Q. Fu, Nanotechnology, 2011, 22, 045704 CrossRef PubMed.
- F. Song, A. K. Soh and Y. L. Bai, Biomaterials, 2003, 24, 3623 CrossRef CAS PubMed.
- D. D. L. Chung, J. Mater. Sci., 2004, 39, 2645 CrossRef CAS.
- H. B. Zhang, W. G. Zheng, Q. Yan, Y. Yang, J. W. Wang and Z. H. Lu, Polymer, 2010, 51, 1191 CrossRef CAS.
- H. Deng, R. Zhang, E. Bilotti, J. Loos and T. Peijs, J. Appl. Polym. Sci., 2009, 113, 742 CrossRef CAS.
- S. Vionnet-Menot, C. Grimaldi, T. Maeder, S. Strassler and P. Ryser, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 064201 CrossRef.
- K. Ogawa, S. Hirano, T. Miyanishi, T. Yui and T. Watanabe, Macromolecules, 1984, 17, 973 CrossRef CAS.
- W. K. Chee, H. N. Lim and N. M. Huang, RSC Adv., 2015, 5, 68014 RSC.
- C. Tang, N. Chen, Q. Zhang, K. Wang, Q. Fu and X. Zhang, Polym. Degrad. Stab., 2009, 94, 12 CrossRef.
- S. Wang, L. Chen and Y. Tong, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 686–696 CrossRef CAS.
- S. F. Wang, L. Shen, Y. J. Tong, L. Chen, I. Y. Phang and P. Q. Lim, Polym. Degrad. Stab., 2005, 90, 123 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |