Coupled cobalt oxide/hollow carbon sphere as an efficient electrocatalyst for the oxygen reduction reaction†
Zongkun Chena,
Dandan Heab,
Xiujuan Xua,
Zhenzhen Liub,
Minghua Huang*a,
Xin Wang*a and
Heqing Jiangb
aInstitute of Materials Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China. E-mail: huangminghua@ouc.edu.cn; wangxinhd@ouc.edu.cn
bInstitute of Biomass Energy and Processing, Chinese Academy of Sciences, Qingdao 266101, P. R. China
First published on 30th March 2016
Abstract
The design of a non-noble-metal electrocatalyst for the oxygen reduction reaction (ORR) is crucial for the renewable energy technologies related to electrochemical energy conversion and storage. Herein, we demonstrate a facile one-pot protocol for the highly selective growth of nanosized cobalt oxide on hollow carbon spheres. The unique structural features enable them to be an efficient non-precious catalyst for the ORR and offer potential applications in the fields of alkaline fuel cells.
Introduction
The oxygen reduction reaction (ORR), the cathodic branch of oxygen electrode reactions with sluggish kinetics, are considered as one of the limiting factors for the application of those renewable energy technologies related to electrochemical energy conversion and storage such as metal–air batteries, fuel cells and chlor-alkali electrolysis etc.1,2 To realize a successful implementation of these technologies, it is necessary to develop efficient ORR electrocatalysts to meet the requirement. Pt-based catalysts are generally believed to be the most promising ones.3 Due to the fact the scarcity and high cost of Pt are impeding the mass commercialization of these technologies, searching for efficient and cheap non-precious-metal catalysts are becoming more preferable over the past years.4–7Recently, transition metal oxide/carbon composite nanomaterials have been successfully used as an efficient alternative to Pt, as a result of the advantages associated with their high activity and low cost.8–13 Co3O4, one of transition metal oxides, exhibits low activity for ORR by itself, albeit high electrocatalytic activity toward OER.11 Dai's group first revealed that Co3O4 nanoparticles grown on reduced mildly oxidized graphene oxide (N-rmGO) exhibited unexpected high ORR activity, due to the synergetic covalent coupling between them, as well as the unique property of N-rmGO.11 Up to now, some carbon materials including carbon nanotube (CNT),12 carbon nanosheet,14 reduced graphene oxide,15 mesoporous carbon,16 and N-doped carbon,17 have been utilized as the carbon precursors to construct the various Co3O4/carbon composites. However, the preparation process is relatively complicated. In most of the cases, the carbon precursors need to be pre-oxidized with oxygen-containing functional groups which can be used nucleating and anchoring sites to couple metal oxide and the carbon precursors with strong interaction.11–14 Moreover, many features including the structural integrity, high electrical conductivity of the carbon materials and a desirable property for charge transport during electrocatalysis may be reduced in the harsh oxidation conditions.18 It would therefore be necessary to develop new and simple strategies to couple Co3O4 and the carbon materials to generate the carbon-based composites.
Among a variety of the carbon materials, hollow carbon sphere (HCS) has attracted significant attention in catalysis, owing to its good conductivity, large surface-to-volume ratios and more accessible active sites on the shell.19–21 It has recently been reported that heteroatom N-, Co–N-, Fe–N-doped HCS could be efficient ORR catalysts.19,21–23 However, the synthetic methods are mainly based on the use of the silica sphere as the hard template to obtain the hollow sphere, which is relatively fussy and time-consuming. To the best of our knowledge, to date metal oxide have not yet exploited to be coupled with the hollow carbon structure for promising ORR catalysts via a facile one-pot method.
Herein we report a simple and useful route to prepare Co3O4 nanoparticles encapsulated inside HCS (denoted as Co3O4/HCS) as ORR electrocatalyst by annealing a hydrothermally treating mixture of non-ionic triblock copolymer P123 (EO20PO70EO20), sodium oleate (SO), Co(NO3)2·6H2O, 2,4-dihydroxybenzoic acid (DA), and hexamethylenetetramine (HMT) at 600 °C in Ar atmosphere.19 The as-prepared Co3O4 nanoparticles are highly selectively grown into the composite due to the presence of the oxygen-containing groups of P123, SO and DA, which could achieve the intimate attachment between Co3O4 and HCS. These features could endow the as-synthesized catalysts with efficient electrocatalytic activities for ORR.
Experimental
Materials
Cobalt(II) nitrate hexahydrate, sodium oleate (SO), 2,4-dihydroxybenzoic acid (DA) and hexamethylenetetramine (HMT) were obtained from Aladdin Chemical Co. Nafion solution (5%) and Pluronic P123 (non-ionic triblock copolymer EO20PO70EO20) were purchased from Sigma-Aldrich (MO, USA). Commercial Pt/C catalyst (20 wt%) was bought from Johnson Matthey Company (Shanghai, China). Isopropanol and other reagents were purchased from Sinopharm Chemical Reagent Co. All chemicals were used as received without any further purification. Water used for preparation of aqueous solution was purified using a Millipore-Q water purification system.Synthesis of Co3O4/HCS and HCS
The synthetic strategy to prepare Co3O4/HCS composite is similar to that recently reported for Pt/HCS.20 First, an aqueous solution A containing 0.375 mM Pluronic P123, 12 mM SO and 3 mM Co(NO3)2·6H2O was prepared. Then, solution B containing 20 mM DA and 8.3 mM HMT was prepared under slow stirring. In the third step, solution A and B were mixed to obtain solution C. After stirring for 10 min, solution C was moved to a Teflon-lined stainless-steel autoclave and preserved for 120 min at 160 °C. The samples were gathered by centrifugation at 9000 min−1 for 10 min (HITACHI CR 22GIII, angle rotor 10 × 50 mL) with deionized water for three times, and maintained at 50 °C under vacuum for 8 h. Finally, the samples were heated to 600 °C or 900 °C with a heating rate of 2 °C min−1 and kept at 600 °C or 900 °C for 3 h with a purge of 30 mL min−1 in argon. The as-prepared composites were denoted as Co3O4/HCS or Co3O4/HCS-900.The procedures for HCS were conducted as described above except without addition of Co(NO3)2·6H2O in the solution A.
Electrode preparation and modification
Glassy carbon electrode (GCE, 3 mm in diameter) and glassy carbon (GC) disk-platinum ring electrode (geometric area: 0.1256 cm2) were polished with alumina powder and then rinsed with deionized water. 6 mg catalysts (HCS or Co3O4/HCS) were redispersed in a 1 mL mixture containing water, isopropanol, and Nafion (v/v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). Before cyclic voltammetry or rotating disk electrode (RDE) experiments, the as-obtained suspension was applied to the GCE with a catalyst loading of 0.25 mg cm−2. The reference commercial Pt/C catalyst ink was prepared to reach 1 mg mL−1 by ultrasonically stirring in a mixture of solvents containing water, isopropanol, and Nafion (5%) (v/v/v = 4
0.1). Before cyclic voltammetry or rotating disk electrode (RDE) experiments, the as-obtained suspension was applied to the GCE with a catalyst loading of 0.25 mg cm−2. The reference commercial Pt/C catalyst ink was prepared to reach 1 mg mL−1 by ultrasonically stirring in a mixture of solvents containing water, isopropanol, and Nafion (5%) (v/v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). The loading of the commercial Pt/C catalyst is 0.042 mg cm−2.
0.1). The loading of the commercial Pt/C catalyst is 0.042 mg cm−2.
Characterization
Transmission electron microscopy (TEM) studies were performed using a Hitachi H-7650 microscope and a G2 microscope operated at 120 kV. The samples for TEM analysis were prepared by dipping the carbon-coated copper grids into the ethanol solution of the products and drying at room temperature. Scanning electron microscopy (SEM) analyses were carried out with Hitachi S-4800 microscopes. XRD patterns were recorded using a Bruker D8 Advance diffractometer operated at 40 kV and 40 mA with a Cu Kα radiation (λ = 1.54178 Å). X-ray photoelectron spectra (XPS) were acquired on an ESCALAB MKII with Mg Kα (hν = 1253.6 eV) as the excitation source. Thermogravimetric (TG) analysis was conducted on a thermogravimetric analyzer, ZRY-2P (China), with a heating rate of 10 °C min−1 under air. Raman spectra were recorded with a Thermo Fisher spectrometer equipped with helium–neon (633 nm) and argon (532 nm) lasers. The laser beam was focused on an area of about 2 mm × 2 mm with power lower than 1 mW.Results and discussion
Preparation and characterization of electrocatalyst
The synthetic strategy to prepare Co3O4/HCS composite is similar to that recently reported for Pt/HCS,20 which involves annealing a hydrothermally treating mixture of P123, SO, Co(NO3)2·6H2O, DA, and HMT at 600 °C in Ar atmosphere by a facile one-pot method. Notably, cobalt species was well anchored by the poly(ethylene oxide) (PEO) part of Pluronic P123, the carboxylate group of sodium oleate and of DA. Such abundant binding site could lead to its highly selective growth on the precursors, which can not only induce the formation of homogeneous Co3O4 without agglomeration, but also provide strong interaction between Co3O4 and HCS in which no complicated pre-oxidation step need to be employed to get oxygen-containing functional groups. Both of them are crucial to the catalytic performance of the Co3O4/HCS composite.X-ray photoelectron spectroscopy (XPS) was first employed to identify the composition of HCS and Co3O4/HCS. The XPS survey spectra of HCS mainly reveals the presence of carbon and oxygen (Fig. 1A), whereas Co3O4/HCS mainly shows the signals of cobalt, carbon and oxygen (Fig. 1C). XPS results show the N content is as low as to be 0.63% for HCS and 0.84% for Co3O4/HCS. It is possible that most of N species from HMT are lost in the calcination process. The Co2p XPS spectra of Co3O4/HCS present two characteristic peaks (780.9 and 796.4 eV) of a Co3O4 phase, indicating the successful formation of Co3O4 nanoparticles (Fig. S1A†).14 As shown in Fig. 1B and D, the high-resolution C1s of both HCS and Co3O4/HCS exhibit four single peaks that are assigned to sp2-hybridized C–C (284.5 eV), C–OH groups (286 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in carbonyl or quinine groups (287.5 eV), and COOH groups (289 eV), respectively.24,25 The presence of the oxygen-containing functional groups may arise from P123, SO and DA, which may provide the nucleating site to anchor Co3O4 nanoparticles. It can be seen that for Co3O4/HCS, the intensity of the peak at 289 eV is lower than that of HCS, indicating the oxygen-containing groups are possibly consumed as anchoring site to couple Co3O4 nanoparticles. Such interaction between them could enable the selective growth of Co3O4 nanoparticles into HCS without any agglomeration. The total content of Co3O4 was calculated to be ca. 4.79 wt% by thermogravimetry under air (Fig. S1B†).
O groups in carbonyl or quinine groups (287.5 eV), and COOH groups (289 eV), respectively.24,25 The presence of the oxygen-containing functional groups may arise from P123, SO and DA, which may provide the nucleating site to anchor Co3O4 nanoparticles. It can be seen that for Co3O4/HCS, the intensity of the peak at 289 eV is lower than that of HCS, indicating the oxygen-containing groups are possibly consumed as anchoring site to couple Co3O4 nanoparticles. Such interaction between them could enable the selective growth of Co3O4 nanoparticles into HCS without any agglomeration. The total content of Co3O4 was calculated to be ca. 4.79 wt% by thermogravimetry under air (Fig. S1B†).
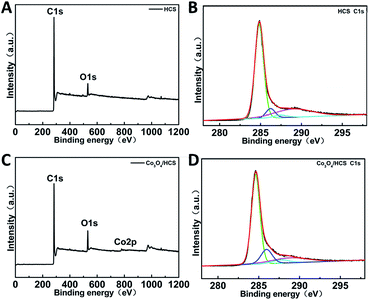 | ||
| Fig. 1 (A) XPS survey spectra and (B) C1s spectra of HCS. (C) XPS survey spectra and (D) C1s spectra of Co3O4/HCS. | ||
Scanning electron microscopy (SEM) was used to investigate the morphology of the hollow carbon-based materials. SEM images of HCS and Co3O4/HCS show the typical sphere structure with a diameter of ca. 200 nm (Fig. S1C and D†). Transmission electron microscopy (TEM) was also used to confirm the successful growth of Co3O4 nanoparticles into HCS. Fig. 2A shows TEM image of HCS. It can be seen that the formation of the hollow carbon structure is clearly observed for HCS, which has a diameter of ca. 200 nm and a shell thickness of ca. 30 nm. To some extent, the obtained HCS structure is disintegrated. Interestingly, upon the growth of Co3O4 nanoparticles into the HCS, the Co3O4/HCS sample highlights the perfect hollow morphology with a diameter of ca. 200 nm and a shell thickness of ca. 30 nm (Fig. 2B–D). Many small Co3O4 nanoparticles with a diameter of ca. 3–5 nm are uniformly encapsulated into HCS. It is notable that there are no individual cobalt oxide nanoparticles or carbon sphere, indicating Co3O4 nanoparticles have been selectively grown into the hollow carbon structures. Another interesting phenomenon is that, even after ultrasonication for half an hour to prepare TEM sample, the morphology are well kept, also indicating the strong interaction between Co3O4 and HCS.
 | ||
| Fig. 2 TEM images of HCS (A) and Co3O4/HCS (B–D). Scale bar: (A–D): 50 nm, 200 nm, 50 nm, and 10 nm, respectively. | ||
The structure of Co3O4 nanoparticles into the hollow carbon was characterized by X-ray diffraction (XRD). Fig. 3A presents XRD patterns of HCS and Co3O4/HCS. Both HCS and Co3O4/HCS show two broad diffraction peaks at 24.5 and 43.1°, which are attributed to the (002) and (100) reflection of graphitic carbon, respectively.22 This result indicates that the graphitic carbon structure has been successfully synthesized, and the obtained carbon species are amorphous and consist of disorder area with low degree of crystallinity.26 For Co3O4/HCS, no cobalt oxide signals is detected upon the encapsulation of Co3O4, indicating the size of cobalt oxide may be comparably small, which is in agreement with TEM results. With increasing the calcination temperature to prepare Co3O4/HCS, it can be expected that the size of Co3O4 nanoparticles will become bigger. XRD patterns of Co3O4/HCS obtained 900 °C shows the typical peaks at 37.5, 45 and 65° assigned to Co3O4 crystalline phase (JCPDS no. 42-1467), as shown in Fig. S2.† Therefore, all of these above-mentioned results support the successful growth of Co3O4 nanoparticles into the hollow carbon structures.
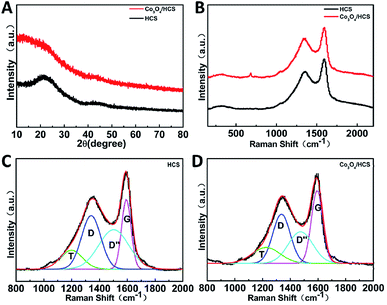 | ||
| Fig. 3 (A) XRD patterns, (B) Raman spectra of HCS and Co3O4/HCS, and (C, D) fitted Raman spectra of HCS and Co3O4/HCS. | ||
The microstructure of the hollow carbon-based materials was further investigated by Raman spectroscopy analysis. Compared to that of HCS, the Raman peak at 682 cm−1 associated with Co3O4 can be only observed for the Co3O4/HCS,27 indicating the successful growth of Co3O4 into the HCS (Fig. 3B), which is consistent with TEM, XPS and XRD results. Both HCS and Co3O4/HCS exhibit the characteristic peaks arising from the carbon materials in the range from 800 to 2000 cm−1 (Fig. 3B).28 The fitting of the spectra are shown in Fig. 3C and D. Both of them show four main peaks corresponding to the disordered graphitic lattice at 1200 cm−1, the disorder-induced D-band at 1335 cm−1, the amorphous carbon structure A-band at 1490 cm−1 and the ideal graphitic lattice G-band at 1595 cm−1, respectively.28 Generally, the intensity ratio value of D- and G-band (IG/ID) is an indicator of the degree of the graphitic ordering in the carbon materials.14 According to the fitting results, the integrated intensity ratio of G- and D-bands (IG/ID) value of Co3O4/HCS is 1.48, which is higher than that of HCS (1.3), indicating Co3O4/HCS exhibits a more ordered graphitic structure than HCS. It is possible that the catalytic effect of cobalt at high temperature can induce such graphitization degree, which is similar to that of other carbon materials promoted by transition metals.29 Such higher degree of graphitization indicates that the composite may serve as a good conductive network for efficient electron transport, which is beneficial for electrocatalysis.
Electrochemical evaluation of Co3O4/HCS for ORR
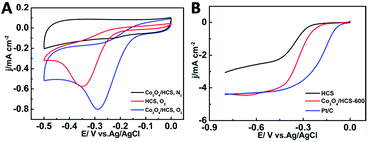
The ORR electrocatalytic performance of different catalysts was first evaluated by cyclic voltammetry. Shown in Fig. 4A are typical CVs of HCS and Co3O4/HCS in 0.1 M KOH with and without O2. In absence of O2, Co3O4/HCS did not show any characteristic voltammogram in the potential range used in the investigation. As expected, in the presence of O2, Co3O4/HCS exhibit a pronounced ORR catalytic reduction peak potential of −0.28 V, which shifts 70 mV more positive than that of HCS. The higher peak current density also indicates the higher catalytic activity for the Co3O4/HCS. In addition, the composite catalyst Co3O4/HCS calcined at higher temperature exhibits better catalytic activity, which is probably related to the changes of particle size and graphitization degree of HCS. The detailed relationship of catalytic activity with graphitization degree of HCS and the size of Co3O4 particles is underway.The catalytic activity of the different catalysts is further confirmed by recording the linear sweep voltammetry (LSV) curves on a rotating disk electrode (RDE), which presents the same sequence of the ORR activity as the above-mentioned catalysts. Fig. 4B shows the ORR polarization curves obtained at a rotation speed of 1600 rpm for HCS, Co3O4/HCS and Pt/C. Compared to HCS, Co3O4/HCS has a more sharp current increase and reach plateau more quickly with a better diffusion-limited current, suggesting better ORR activity for Co3O4/HCS. Moreover, Co3O4/HCS clearly displays more positive onset potential and half-wave potential than HCS, indicating an enhanced ORR activity has been obtained for Co3O4/HCS. It also exhibits higher limiting current density. It was also noted that the onset potential and half-wave potential for ORR at Co3O4/HCS were better than that of cobalt–N-doped HCS by hard template method,21 although lower than that at the commercial Pt/C and those at the few state-of-the-art Co3O4/N-rmGO and cobalt oxide/CNT.11,12 In comparing these results, it becomes evident that the well-behaved electrocatalysis behaviour of Co3O4/HCS should originate from the uniform dispersion of Co3O4 nanoparticles, high graphitization of HCS, and strong interaction between Co3O4 and HCS.
In order to obtain further information on the electrocatalytic system, RDE voltammetry technique was used for HCS and Co3O4/HCS at different rotation speeds. It can be seen that for Co3O4/HCS, the limiting current density increase gradually with increasing the rotation rate and the potential-independent plateau currents are present at all rotation rate (Fig. 5A). As expected, the good linearity and parallelism are obtained between the inverse of the limiting current and the inverse of the square root of the rotating rate at different potential values (Fig. 5B, Koutecky–Levich plot). The calculated number of electrons involved in the reduction of O2 from the slope of the Koutecky–Levich plot is found to be 3.7–3.8 in the potential range of −0.5 to −0.7 V. It is obvious that this number is close to theoretical value 4 and the ORR is dominated by a 4e reduction process to OH−. In the control experiment, HCS exhibits low ORR activity with a slow current increase and no current plateau (Fig. S3A†). As seen from the corresponding Koutecky–Levich plot (Fig. S3B†), the number of electron transfer is calculated to be 2.7 at −0.7 V, thus suggesting the HCS catalyst without Co3O4 favour a dominated two-electron reduction pathway to HO2−. According to these above results, Co3O4/HCS is more favourable than that of HCS for ORR.
A rotating disk-ring electrode (RRDE) measurement was employed to determine the quantity of peroxide species (HO2−) that was generated by a two-electron reduction process of O2 at the disk electrode, which provides further supports for the effectiveness of Co3O4/HCS for the proposed four-electron reduction of oxygen. Fig. 5C and D shows the ORR polarization curves recorded on the RRDE at a rotation speed of 1600 rpm for HCS and Co3O4/HCS. It can be seen that Co3O4/HCS exhibits higher disk current and lower ring current than that of HCS, indicating the enhanced ORR activity for the composite materials. For Co3O4/HCS, its ring current from HO2− oxidation is much smaller than the disk current from oxygen reduction, which indicates a small percentage of HO2− were produced in the ORR process catalyzed by the catalyst. The yield of HO2− is calculated to be 21.5% at −0.65 V. The calculated number of electrons involved in the reduction of O2 was found to be about 3.6 for Co3O4/HCS and 2.7 for HCS, which is almost identical to that acquired from the Koutecky–Levich plot (vide supra). This result indicates that the reduction of oxygen at Co3O4/HCS mainly supports the four-electron pathway to produce a low yield of HO2−.
The poisoning effect of methanol for ORR has been conducted. As shown in Fig. S4,† the curves for the Co3O4/HCS present no obvious change in the absence and presence of 1.0 M methanol in O2-saturated 0.1 M KOH. In contrast, the typical inverse methanol oxidation peaks can be observed for Pt/C with the addition of methanol in O2-saturated 0.1 M KOH. These results reveal that Co3O4/HCS has a better methanol tolerance towards ORR than the commercial Pt/C. The durability of the Co3O4/HCS catalyst was evaluated by conducting the repeated potential dynamic cycling durability tests for 3500 cycles in O2-saturated 0.1 M KOH solution. As presented in Fig. S5,† after 3500 cycles, about 30 mV negative shift of half-wave potential is shown for Co3O4/HCS which is close to that of a commercial Pt/C. Although the current densities for both Co3O4/HCS and Pt/C decrease with time, Co3O4/HCS shows a slower decrease, indicating a higher stability for Co3O4/HCS, which further confirms Co3O4/HCS is an efficient catalyst for ORR.
Conclusions
In summary, nanosized Co3O4 and HCS has been deliberately coupled to be as an efficient ORR catalyst by a facile one-pot method. Since the abundant oxygen-containing functional groups within the carbon precursor could be the nucleating and anchoring sites, the Co3O4 nanoparticles are well controlled to grow selectively within HCS, which can avoid the aggregation of them. Such intimate coupling between Co3O4 and HCS lead to facile electron accessibility and thus enhanced catalytic activity for ORR, which promotes the development of new and efficient ORR catalysts with low cost for alkaline fuel cell. Besides, due to the presence of reactive oxygen-containing groups within HCS and Co3O4/HCS, they could be easily assembled or integrated on solid surfaces for future application in nanodevices. Furthermore, the simple strategy could prove to be universal for other transition metal oxide (e.g. Cu, Ni, Fe and Mn and so on) coupled with HCS for various applications in the fields of electrochemical energy conversion and storage.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21405145), China Postdoctoral Science Foundation (2014M551955), Qingdao Science and Technology Plan (14-2-4-100-jch), Shandong and Qingdao Postdoctoral Science Foundation, the Open Funds of the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201509), and Seed Fund from Ocean University of China, and Fundamental Research Funds for the Central Universities.Notes and references
- J. T. Zhang, Z. H. Zhao, Z. H. Xia and L. M. Dai, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS PubMed.
- X. M. Ge, A. Sumboja, D. Wuu, T. An, B. Li, F. W. T. Goh, T. S. A. Hor, Y. Zong and Z. L. Liu, ACS Catal., 2015, 5, 4643–4667 CrossRef CAS.
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443–447 CrossRef CAS PubMed.
- B. Y. Xia, Y. Yan, X. Wang and X. W. Lou, Mater. Horiz., 2014, 1, 379–399 RSC.
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. Lou and X. Wang, Nat. Energy, 2016, 1, 15006–15013 CrossRef.
- Y. Z. Dong and J. L. Li, Chem. Commun., 2015, 51, 572–575 RSC.
- B. B. Lv, T. N. Ye, L. H. Gong, K. X. Wang, J. Su, X. H. Li and J. S. Chen, Chem. Mater., 2015, 27, 544–549 CrossRef.
- W. Xia, R. Q. Zou, L. An, D. G. Xia and S. J. Guo, Energy Environ. Sci., 2015, 8, 568–576 CAS.
- S. J. Guo, S. Zhang, L. H. Wu and S. H. Sun, Angew. Chem., Int. Ed., 2012, 51, 11770–11773 CrossRef CAS PubMed.
- Y. H. Su, H. L. Jiang, Y. H. Zhu, X. L. Yang, J. H. Shen, W. J. Zou, J. D. Chen and C. Z. Li, J. Mater. Chem. A, 2014, 2, 7281–7287 CAS.
- Y. Y. Liang, Y. Y. Li, H. L. Wang, J. G. Zhou, J. Wang, T. Regier and H. J. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- Y. Y. Liang, H. L. Wang, P. Diao, W. Chang, G. S. Hong, Y. G. Li, M. Gong, L. M. Xie, J. G. Zhou, J. Wang, T. Z. Regier, F. Wei and H. J. Dai, J. Am. Chem. Soc., 2012, 134, 15849–15857 CrossRef CAS PubMed.
- Y. G. Wang, L. Cheng, F. Li, H. M. Xiong and Y. Y. Xia, Chem. Mater., 2007, 19, 2095–2101 CrossRef CAS.
- H. L. Wang, N. Mao, J. Shi, Q. G. Wang, W. H. Yu and X. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 2882–2890 CAS.
- M. Wang, J. Huang, M. Wang, D. Zhang, W. Zhang, W. Li and J. Chen, Electrochem. Commun., 2013, 34, 299–303 CrossRef.
- Y. Wang, X. Cui, L. Chen, C. Wei, F. Cui, H. Yao, J. Shi and Y. Li, Dalton Trans., 2014, 4163–4168 RSC.
- G. Zhang, C. Li, J. Liu, L. Zhou, R. Liu, X. Han, H. Huang, H. Hu, Y. Liu and Z. Kang, J. Mater. Chem. A, 2014, 2, 8184–8189 CAS.
- Y. G. Li, W. Zhou, H. L. Wang, L. M. Xie, Y. Y. Liang, F. Wei, J. C. Idrobo, S. J. Pennycook and H. J. Dai, Nat. Nanotechnol., 2012, 7, 394–400 CrossRef CAS PubMed.
- Y. Wang, A. G. Kong, X. T. Chen, Q. P. Lin and P. Y. Feng, ACS Catal., 2015, 5, 3887–3893 CrossRef CAS.
- G. H. Wang, J. Hilgert, F. H. Richter, F. Wang, H. J. Bongard, B. Spliethoff, C. Weidenthaler and F. Schüth, Nat. Mater., 2014, 13, 293–300 CrossRef CAS PubMed.
- H. Wang, X. J. Bo, A. X. Wang and L. P. Guo, Electrochem. Commun., 2013, 36, 75–79 CrossRef CAS.
- J. Sanetuntikul, T. Hang and S. Shanmugam, Chem. Commun., 2014, 50, 9473–9476 RSC.
- C. L. Han, S. P. Wang, J. Wang, M. M. Li, J. Deng, H. R. Li and Y. Wang, Nano Res., 2014, 7, 1809–1819 CrossRef CAS.
- A. Ganguly, S. Sharma, P. Papakonstantinou and J. Hamilton, J. Phys. Chem. C, 2011, 115, 17009–17019 CAS.
- R. S. Zong, Y. H. Qin, D. F. Niu, J. W. Tian, X. S. Zhang, X. G. Zhou, S. G. Sun and W. K. Yuan, J. Power Sources, 2013, 22, 192–199 CrossRef.
- R. M. Li, A. M. Cao, Y. J. Zhang, G. Li, F. Jiang, S. M. Li, D. Q. Chen, C. R. Wang, J. C. Ge and C. Y. Shu, ACS Appl. Mater. Interfaces, 2014, 6, 20574–20578 CAS.
- J. P. Liu, J. Jiang, C. W. Chen, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- J. Wang, H. Y. Liu, J. Y. Diao, X. M. Gu, H. H. Wang, J. F. Rong, B. N. Zong and D. S. Su, J. Mater. Chem. A, 2015, 3, 2305–2313 CAS.
- M. Sevila and A. B. Fuertes, Carbon, 2006, 44, 468–474 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Rotating disk electrode (RDE) and rotating-ring disk electrode (RRDE) experiment; additional XPS spectrum, XRD patterns and RDE results. See DOI: 10.1039/c6ra04714a |
| This journal is © The Royal Society of Chemistry 2016 |