DOI:
10.1039/C6RA03070J
(Paper)
RSC Adv., 2016,
6, 34146-34152
tert-Butyl nitrite mediated azo coupling between anilines and imidazoheterocycles†
Received
2nd February 2016
, Accepted 29th March 2016
First published on 30th March 2016
Abstract
A tBuONO mediated practical and efficient method for the synthesis of azo imidazoheterocycles with broad functionalities has been achieved by the reaction between imidazoheterocycles and anilines at room temperature in goods yields. Azo indolizines were also prepared by employing this methodology. Mild reaction conditions, tolerance of wide functionalities, operational simplicity and easy purification of the products are the notable advantages of the current protocol.
Introduction
Azoheterocycles are well known for their uses as organic dyestuffs, pharmaceutical agents and food additives.1 Besides traditional utilities, these scaffolds have been successfully employed as photodissociable ligands in molecular switches and machines owing to their light driven cis/trans isomerisation2 (Fig. 1). A few azoheterocycles exhibit a potential role in various fields of the electronic industry, namely, non-linear optics,3 liquid crystal technology4 and photodynamic therapy.5 Due to diverse applications of these compounds, continuous efforts have been made to device new synthetic methods for azoheterocyclic compounds.1a,2a,6
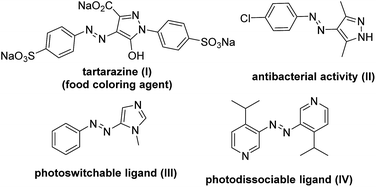 |
| | Fig. 1 Important azoheteroarene scaffolds. | |
Imidazo[1,2-a]pyridine moiety displays an extensive array of biological activities such as antitumor, antiparasitic, anti-inflammatory, antiviral, hypnotic, analgesic, antipyretic, etc.7 Some marketed drugs like olprinone, alpidem, zolpidem, saripidem etc. contain this fused bicyclic 5–6 nitrogenous heterocycle. Imidazo[1,2-a]pyridine derivatives are also found to be useful in material sciences.8 Accordingly, much efforts have been made for the synthesis and functionalization of imidazopyridine scaffold.9 For the last few years our group is also actively engaged in developing strategies for synthesis10 and functionalization of imidazoheterocycles.11
Based on our experiences we envisioned that incorporation of azo functionality in the imidazo[1,2-a]pyridine ring could impart marked biological properties as well as photoresponsive soft materials based applications. Generally azoimidazo[1,2-a]pyridines are synthesized through diazo coupling using conc. HCl and NaNO2.6e Herein, we report a straightforward and environmentally benign protocol for the synthesis of azoimidazo[1,2-a]pyridines by the reaction between anilines and imidazo[1,2-a]pyridines using tBuONO in EtOH at room temperature (Scheme 1).
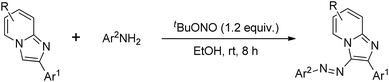 |
| | Scheme 1 Synthesis of (E)-3-(aryldiazenyl)imidazo[1,2-a]pyridines. | |
Results and discussion
Our effort was initiated by the reaction between p-toluidine (2a, 1.1 equiv.) and 8-methyl-2-phenylimidazo[1,2-a]pyridine (1a) in presence of tBuONO (1.2 equiv.) at room temperature in DMSO affording the diazo compound 3aa in 68% (Table 1, entry 1). Next we checked the effect of various solvents like DMF, CH3CN, THF, 1,4-dioxane, DCM, MeOH and EtOH (Table 1, entries 2–8). Among them, EtOH was found to be the best solvent to furnish the desired product with 88% yield after 8 h (Table 1, entry 8) probably due to fact that EtOH is a polar protic solvent which helps to stabilize the diazonium ion. Prolonged reaction time did not improve the yield. Next we increased the amount of p-toluidine (2a) and tBuONO, but no significant increase in yield was seen to occur (Table 1, entry 9). Only a trace amount of product was obtained when the reaction was performed at 0 °C for 24 h (Table 1, entry 10).
Table 1 Optimization of the reaction conditionsa
|
| Entry |
Solvent |
2a (equiv.) |
tBuONO (equiv.) |
Temp (°C) |
Time (h) |
Yield (%) |
| Reaction conditions: all reactions were carried out on 0.20 mmol scale in solvent (1.5 mL). No reaction. |
| 1 |
DMSO |
1.1 |
1.2 |
rt |
24 |
68 |
| 2 |
DMF |
1.1 |
1.2 |
rt |
24 |
54 |
| 3 |
CH3CN |
1.1 |
1.2 |
rt |
24 |
43 |
| 4 |
THF |
1.1 |
1.2 |
rt |
24 |
63 |
| 5 |
1,4-Dioxane |
1.1 |
1.2 |
rt |
24 |
56 |
| 6 |
DCM |
1.1 |
1.2 |
rt |
24 |
nrb |
| 7 |
MeOH |
1.1 |
1.2 |
rt |
24 |
76 |
| 8 |
EtOH |
1.1 |
1.2 |
rt |
8 |
88 |
| 9 |
EtOH |
1.5 |
2 |
rt |
24 |
88 |
| 10 |
EtOH |
1.1 |
1.2 |
0 |
24 |
Trace |
With the optimized reaction conditions in hand, the substrate scope of the present protocol was explored and the results are summarized in Scheme 2. A variety of anilines having both electron donating substituents like –Me, –OMe and electron withdrawing groups like –CN, –NO2, –CO2Et, –COMe, –COPh were studied under the present reaction conditions. It is notable that electron-deficient anilines gave better yields (3ad–ah) compared to electron-rich anilines (3aa and 3ab) as diazonium salts bearing electron withdrawing groups are stronger electrophiles than electron-rich diazonium salts. The anilines having different halogens like –Cl, –F and –I successfully reacted with imidazopyridine to give the corresponding products in significant yields (3ai–ak). Importantly, the reaction between 8-methyl-2-phenylimidazo[1,2-a]pyridine (1a) and 2-aminobenzenesulfonamide (2m) afforded ethyl benzenesulfonate substituted azo derivative 3am.
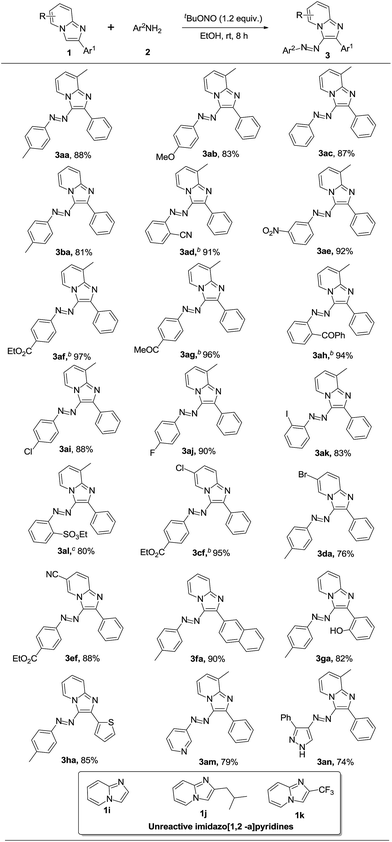 |
| | Scheme 2 Substrates scopea, areaction conditions: 1 (0.20 mmol), 2 (0.22 mmol) and tBuONO (0.24 mmol) in EtOH (1.5 mL) at rt for 8 h. bReactions completed in 2 h. ctBuONO (0.4 mmol). | |
Next, we checked the scope of imidazopyridines having different substituents like –Cl, –Br and –CN on the pyridine ring as shown in Scheme 2. All of them gave the azoimidazopyridines (3cf, 3da and 3ef) with good to excellent yields. Imidazopyridines having different aryl (3fa and 3ga) and heteroaryl (3ha) substitutions at C-2 position furnished the products with good yields. To our delight, heteroaryl amines such as aminopyrazoles and aminopyridines also worked well under the present reaction conditions (3an and 3ao). However, 2,3-unsubstituted imidazo[2,1-a]pyridine (1i), 2-isobutyl and CF3-substituted imidazo[2,1-a]pyridine (1j and 1k) did not afford the desired products.
The stereochemistry of 8-methyl-2-phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3aa) was further confirmed by X-ray single crystallographic analysis (Fig. 2).12
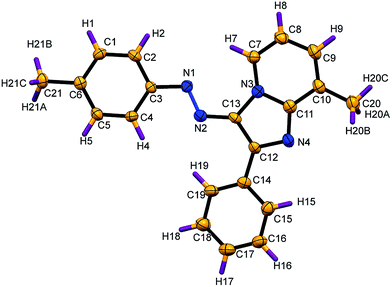 |
| | Fig. 2 X-ray crystal structure of 3aa (thermal ellipsoids are drawn at 30% probability level). | |
Next we extended our protocol to other imidazoheterocycles like benzo[d]imidazo[2,1-b]thiazole and imidazo[2,1-b]thiazole (Scheme 3). Pleasingly, the corresponding azo products were obtained with excellent yields (5aa–ca).
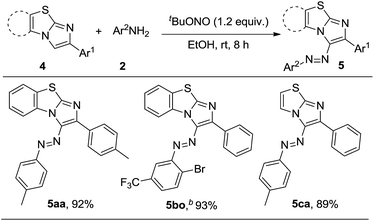 |
| | Scheme 3 Substrate scope of imidazoheterocyclesa, areaction conditions: 4 (0.20 mmol), 2 (0.22 mmol) and tBuONO (0.24 mmol) in EtOH (1.5 mL) at rt for 8 h. bReaction completed in 1 h. | |
Other than imidazoheterocycles, we were successful to install the azo functionality in indolizine moiety regioselectively (Scheme 4). Indolizines bearing –CO2Me and –CN successfully reacted with differently substituted anilines to give the desired products in very good yields (7af and 7bg).
 |
| | Scheme 4 Synthesis of azo indolizinesa, areaction conditions: 0.20 mmol of 6 and 0.22 mmol of 2 in EtOH (1.5 mL) in the presence of tBuONO (0.24 mmol) at rt for 4 h. | |
The present protocol is also applicable for the intramolecular azo coupling. Benzo[e]pyrrolo[2,1-c]-1,2,4-benzotriazine13 9 was obtained with 78% yield (Scheme 5). It is worthy to mention that benzotriazine 9 is an analogue of indole[1,2-c]-1,2,4-benzotriazine derivatives which exhibit potent antifungal activities against five phytopathogenic fungi.14
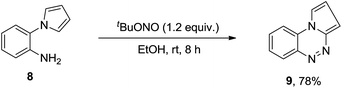 |
| | Scheme 5 Synthesis of benzo[e]pyrrolo[2,1-c][1,2,4]triazine. | |
Practical applicability of the present methodology was examined by carrying out the gram-scale reaction in laboratory setup (Scheme 6). The reaction afforded the azo imidazo[1,2-a]pyridine (3aa) without significant decrease in yield (84%).
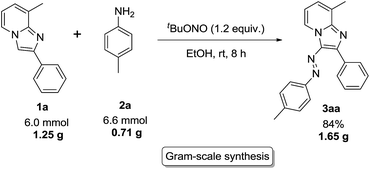 |
| | Scheme 6 Gram-scale reaction.a | |
A plausible mechanism is outlined in Scheme 7. First, aniline 2a is converted to diazonium intermediate15 A in presence of tBuONO which subsequently reacted with imidazopyridine 1a at 3-postion. Finally the azo compound 3aa is formed via deprotonation of intermediate B. The presence of aryl group at 2-positon of imidazopyridine ring is necessary to stabilise the generated positive charge at 2-positon of the intermediate B. Therefore, 2-alkyl substituted imidazopyridine (1j) is inert under the present reaction conditions.
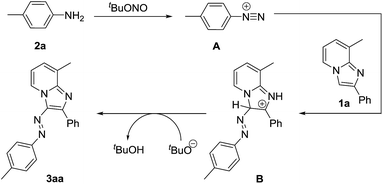 |
| | Scheme 7 Plausible reaction pathway. | |
Conclusions
In summary, we have developed a convenient method for the synthesis of azo imidazo[1,2-a]pyridines. A variety of (E)-3-(aryldiazenyl)imidazo[1,2-a]pyridines with wide range of functionalities were synthesized in good to excellent yields under neutral conditions. Intriguingly, the protocol is also applicable to other heterocycles like imidazo[2,1-b]thiazoles, benzo[d]imidazo[2,1-b]thiazoles and indolizines. The developed reaction procedure shows broad substrates scope, uses safe reagents and solvent, clean reaction, and product isolation by simple filtration making it a useful and preparative method. We believe it will gain much importance in academia as well as industry for the synthesis of functionalized azoheterocycles.
Experimental section
General information
All reagents were purchased from commercial sources and used without further purification, unless otherwise mentioned. Petroleum ether (PE) refers to the fraction of petroleum boiling between 60 and 80 °C. All reactions were carried out in oven-dried glassware. All solvents were dried and distilled before use. TLC was monitored on aluminium backed Silica Gel 60 (HF254) 0.25 mm plates (Merck) using various organic solvent mixtures. Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded on a FTIR instrument. 1H and 13C NMR spectra were acquired on spectrometers operating at 300 or 400 or 500 MHz and 75 or 100 or 125 MHz respectively, using CDCl3 (δ = 7.26 ppm for the 1H NMR and δ = 77.16 ppm for the 13C NMR) or DMSO-d6 (δ = 2.50 ppm for the 1H NMR and δ = 39.50 ppm for the 13C NMR) solvent. High resolution mass spectra were obtained using Qtof Micro YA263 instrument. X-ray single crystal data were collected using MoKα (λ = 0.7107 Å) radiation on a SMART APEX diffractometer equipped with CCD area detector. Data collection, data reduction, structure solution/refinement were carried out using the software package of SMART APEX. Imidazoheterocycles were prepared by our reported methods,10c and indolizine heterocycles were prepared by Hu et al. report.16
Typical experimental procedure for the synthesis of (E)-8-methyl-2-phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3aa)
A mixture of p-toluidine 2a (0.22 mmol, 21 mg) and tBuONO (0.24 mmol, 25 mg, 29 μL) in EtOH (1.5 mL) was taken in a round bottom flask and was stirred for 3 min at room temperature. To this solution 8-methyl-2-phenylimidazo[1,2-a]pyridine 1a (0.20 mmol, 42 mg) was added and allowed to stir for 8 h (TLC). The orange precipitate was collected, washed with EtOH (5 mL) and 10% ethyl acetate/petroleum ether (3 mL). The solid product was dried under vacuum to afford pure (E)-8-methyl-2-phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine 3aa in 88% yield.
Synthesis of benzo[e]pyrrolo[2,1-c][1,2,4]triazine (9). To a solution of 2-(1H-pyrrol-1-yl)aniline 8 (0.20 mmol) in EtOH (1 mL) was added tBuONO (0.24 mmol, 25 mg, 29 μL) and the reaction mixture was stirred at room temperature for 8 h (TLC). The yellow precipitate was collected, washed with EtOH (5 mL) and 10% ethyl acetate/petroleum ether (3 mL). The solid product was dried under vacuum to furnish pure benzo[e]pyrrolo[2,1-c][1,2,4]triazine 9.
(E)-8-Methyl-2-phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3aa). Orange solid (88%, 57 mg), mp 104–105 °C. IR (KBr) νmax: 3443, 3022, 2465, 1599, 1524, 1477, 1445, 1350, 1252 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.81 (d, J = 6.8 Hz, 1H), 8.46 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 8.0 Hz, 2H), 7.54 (t, J = 7.6 Hz, 2H), 7.48–7.44 (m, 1H), 7.30–7.22 (m, 3H), 6.91 (t, J = 6.8 Hz, 1H), 2.72 (s, 3H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 152.0, 149.7, 146.0, 139.7, 133.5, 132.5, 130.1, 129.9, 129.1, 128.5, 128.4, 127.4, 127.2, 122.0, 115.1, 21.5, 17.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H19N4: 327.1610; found: 327.1602.
(E)-3-((4-Methoxyphenyl)diazenyl)-8-methyl-2-phenylimidazo[1,2-a]pyridine (3ab). Orange solid (83%, 57 mg), mp 106–108 °C. IR (KBr) νmax: 3566, 3431, 3408, 3137, 2825, 1603, 1582, 1497, 1439, 1375, 1308, 1250 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.80 (d, J = 6.4 Hz, 1H), 8.44 (d, J = 7.6 Hz, 2H), 7.80 (d, J = 8.8 Hz, 2H), 7.53 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.2 Hz, 1H), 7.28 (t, J = 6.8 Hz, 1H), 7.00–6.95 (m, 3H), 3.88 (s, 3H), 2.78 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 161.2, 148.0, 144.7, 132.3, 132.1, 130.3, 129.4, 129.2, 128.5, 127.10, 127.06, 123.8, 115.6, 114.5; 55.7, 17.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H19N4O: 343.1559; found: 343.1555.
(E)-8-Methyl-2-phenyl-3-(phenyldiazenyl)imidazo[1,2-a]pyridine (3ac). Orange solid (87%, 54 mg), mp 98–100 °C. IR (KBr) νmax: 3059, 3028, 2395, 1618, 1591, 1579, 1525, 1483, 1471, 1444, 1379, 1350, 1290, 1250 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.86 (d, J = 6.8 Hz, 1H), 8.44 (d, J = 7.2 Hz, 2H), 7.87 (d, J = 7.6 Hz, 2H), 7.54–7.46 (m, 5H), 7.39 (t, J = 7.2 Hz, 1H), 7.29 (d, J = 6.8 Hz, 1H), 6.97 (t, J = 6.8 Hz, 1H), 2.74 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 153.8, 150.2, 146.0, 133.1, 130.2, 129.6, 129.4, 129.32, 129.26, 129.0, 128.5, 127.4, 127.2, 122.1, 115.5, 17.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H17N4: 313.1453; found: 313.1447.
(E)-2-Phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3ba). Orange solid (81%, 51 mg), mp 103–104 °C. IR (KBr) νmax: 3049, 3026, 1626, 1597, 1570, 1552, 1445, 1385, 1350, 1246 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.97 (d, J = 6.8 Hz, 1H), 8.45–8.43 (m, 2H), 7.78–7.74 (t, 3H), 7.55–7.51 (m, 2H), 7.48–7.44 (m, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.03 (t, J = 6.8 Hz, 1H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 152.0, 150.3, 146.0, 139.9, 133.4, 132.3, 130.1, 130.0, 129.4, 129.27, 129.25, 128.6, 122.1, 117.6, 115.2, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H17N4: 313.1453; found: 313.1447.
(E)-2-((8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)benzonitrile (3ad). Orange solid (91%, 61 mg), mp 141–142 °C. IR (KBr) νmax: 3496, 3061, 2916, 2393, 2289, 2225, 1925, 1619, 1522, 1478, 1384, 1282, 1242, 1212 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.02 (s, 1H), 8.42 (d, J = 6.0 Hz, 2H), 7.88 (d, J = 4.4 Hz, 1H), 7.77 (d, J = 4.8 Hz, 1H), 7.64 (d, J = 7.2 Hz, 1H), 7.54–7.47 (m, 3H), 7.43–7.38 (m, 2H), 7.16 (d, J = 6.4 Hz, 1H), 2.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 156.0, 152.9, 147.1, 133.6, 133.2, 132.6, 131.1, 130.4, 130.0, 128.73, 128.66, 128.4, 127.6, 118.3, 117.0, 115.6, 112.0, 17.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H16N5: 338.1406; found: 338.1399.
(E)-8-Methyl-3-((3-nitrophenyl)diazenyl)-2 phenylimidazo[1,2-a]pyridine (3ae). Orange solid (92%, 66 mg), mp 152–153 °C. IR (KBr) νmax: 3281, 3099, 1618, 1526, 1508, 1474, 1456, 1416, 1350, 1319, 1286, 1246 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.80 (d, J = 6.8 Hz, 1H), 8.60 (t, J = 6.8 Hz, 1H), 8.40 (d, J = 8.0 Hz, 2H), 8.17–8.16 (m, 1H), 8.09 (d, J = 7.2 Hz, 1H), 7.62–7.46 (m, 4H), 7.35 (d, J = 7.2 Hz, 1H), 7.02 (t, J = 7.2 Hz, 1H), 2.72 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 154.7, 152.8, 149.3, 147.1, 133.1, 132.5, 130.2, 129.98, 129.95, 129.8, 128.7, 127.9, 127.6, 126.8, 122.8, 117.4, 116.0, 16.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N5O2: 358.1304; found: 358.1298.
(E)-Ethyl-4-((8-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)benzoate (3af). Red solid (97%, 75 mg), mp 126–128 °C. IR (KBr) νmax: 3369, 2980, 2902, 2841, 2446, 1711, 1599, 1477, 1273, 1248 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.85 (d, J = 6.8 Hz, 1H), 8.44–8.42 (m, 2H), 8.15 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 8.4 Hz, 2H), 7.55–7.45 (m, 3H), 7.32 (d, J = 7.2 Hz, 1H), 6.99 (t, J = 7.2 Hz, 1H), 4.41 (q, J = 6.8 Hz, 2H), 2.72 (s, 3H), 1.43 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.4, 156.8, 152.1, 146.9, 133.3, 132.9, 130.8, 130.2, 129.6, 128.6, 127.7, 127.5, 121.8, 115.8, 61.2, 16.9, 14.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H21N4O2: 385.1665; found: 385.1660.
(E)-1-(4-((8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)phenyl)ethanone (3ag). Orange solid (96%, 68 mg), mp 114–116 °C. IR (KBr) νmax: 3854, 3838, 3821, 3749, 3736, 3674, 3651, 3616, 3061, 2991, 2918, 1680, 1651, 1595, 1560, 1541, 1524, 1512, 1491, 1477, 1460, 1443, 1377, 1350, 1292, 1263, 1248 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.85 (d, J = 6.8 Hz, 1H), 8.43 (d, J = 7.2 Hz, 2H), 8.06 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 8.4 Hz, 2H), 7.55–7.46 (m, 3H), 7.33 (d, J = 6.8 Hz, 1H), 7.00 (t, J = 6.8 Hz, 1H), 2.72 (s, 3H), 2.64 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 197.4, 156.6, 152.0, 146.7, 136.4, 133.1, 132.8, 130.1, 130.0, 129.6, 129.5, 128.5, 127.5, 127.4, 121.8, 115.7, 26.8, 16.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H19N4O: 355.1559; found: 355.1552.
(E)-(2-((8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)phenyl) (phenyl)methanone (3ah). Orange solid (94%, 78 mg), mp 111–112 °C. IR (KBr) νmax: 3309, 3134, 3061, 2918, 2367, 2338, 1901, 1663, 1589, 1522, 1478, 1441, 1383, 1314 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.61 (d, J = 6.8 Hz, 1H), 8.38–8.36 (m, 2H), 7.92–7.89 (m, 3H), 7.66–7.59 (m, 2H), 7.55–7.47 (m, 5H), 7.43–7.39 (m, 2H), 7.21 (d, J = 6.8 Hz, 1H), 6.57 (t, J = 7.2 Hz, 1H), 2.69 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 198.2, 151.8, 146.0, 138.4, 137.8, 133.4, 132.7, 132.6, 131.2, 130.3, 130.1, 129.8, 129.7, 129.4, 128.9, 128.7, 128.6, 127.2, 127.0, 116.3, 115.6, 17.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H21N4O: 417.1715; found: 417.1712.
(E)-3-((4-Chlorophenyl)diazenyl)-8-methyl-2-phenylimidazo[1,2-a]pyridine (3ai). Orange solid (88%, 61 mg), mp 100–101 °C. IR (KBr) νmax: 3043, 2918, 2849, 2422, 1618, 1583, 1572, 1522, 1472, 1445, 1383, 1375, 1350, 1308, 1294, 1252 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.82 (d, J = 6.8 Hz, 1H), 8.41 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 6.8, 2H), 7.55–7.51 (m, 2H), 7.48–7.44 (m, 3H), 7.31 (d, J = 6.8, 1H), 6.98 (t, J = 6.8 Hz, 1H), 2.73 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 152.5, 151.1, 146.5, 134.8, 133.4, 130.2, 129.5, 129.4, 129.1, 128.6, 127.7, 127.3, 123.2, 115.5, 16.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16ClN4: 347.1063; found: 347.1066.
(E)-3-((4-Fluorophenyl)diazenyl)-8-methyl-2-phenylimidazo[1,2-a]pyridine (3aj). Orange solid (90%, 59 mg), mp 102–103 °C. IR (KBr) νmax: 3130, 3053, 3032, 2229, 2129, 1587, 1477, 1445, 1425, 1379, 1348, 1294, 1247 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.75 (d, J = 6.8 Hz, 1H), 8.42–8.39 (m, 2H), 7.83–7.79 (m, 2H), 7.55–7.51 (m, 2H), 7.48–7.43 (m, 1H), 7.26–7.24 (m, 1H), 7.18–7.13 (m, 2H), 6.92 (t, J = 7.2 Hz, 1H), 2.71 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 163.2 (d, 1JCF = 249 Hz), 150.5, 150.4 (d, 4JCF = 2 Hz), 146.3, 133.5, 132.4, 130.1, 129.2, 128.6, 128.5, 127.5, 127.1, 123.7 (d, 2JCF = 9 Hz), 116.1 (d, 2JCF = 23 Hz), 115.2, 16.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16FN4: 331.1359; found: 331.1368.
(E)-3-((2-Iodophenyl)diazenyl)-8-methyl-2-phenylimidazo[1,2-a]pyridine (3ak). Orange solid (83%, 73 mg), mp 111–112 °C. IR (KBr) ν: 3117, 2916, 1522, 1481, 1373, 1238 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.13 (d, J = 6.4 Hz, 1H), 8.44 (d, J = 8.0 Hz, 2H), 8.00 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.55–7.52 (m, 2H), 7.49–7.41 (m, 2H), 7.36 (d, J = 7.2 Hz, 1H), 7.06 (t, J = 7.6 Hz, 2H), 2.74 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 153.1, 152.1, 146.9, 139.8, 133.4, 133.0, 130.4, 130.3, 129.7, 129.5, 129.1, 128.7, 128.6, 127.5, 117.2, 116.0, 101.5, 16.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16IN4: 439.0420; found: 439.0436.
(E)-Ethyl-2-((8-methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)benzenesulfonate (3al). Compound 3al was obtained as described for 3aa by using 2-aminobenzenesulfonamide 2l (0.22 mmol, 38 mg), tBuONO (0.4 mmol, 42 mg, 48 μL), 8-methyl-2-phenylimidazo[1,2-a]pyridine 1a (0.20 mmol, 42 mg). Orange solid (80%, 67 mg), mp 182–183 °C. IR (KBr) νmax: 3089, 1586, 1510, 1382, 1346, 1243 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.27 (d, J = 6.4 Hz, 1H), 8.44 (d, J = 7.2 Hz, 2H), 8.13 (d, J = 7.6 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.72 (t, J = 7.2 Hz, 1H), 7.56–7.41 (m, 5H), 7.13 (t, J = 7.2 Hz, 1H), 4.18 (q, J = 6.8 Hz, 2H), 2.75 (s, 3H), 1.25 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, DMSO-d6): δ 143.8, 132.9, 131.7, 131.1, 130.6, 130.1, 129.9, 129.8, 129.4, 128.8, 128.4, 127.7, 127.0, 125.1, 123.3, 117.1, 114.2, 56.1, 18.6, 16.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H21N4O3S: 421.1334; found: 421.1327.
(E)-Ethyl-4-((6-chloro-2-phenylimidazo[1,2-a]pyridine-3-yl)diazenyl)benzoate (3cf). Orange solid (95%, 78 mg), mp 140–141 °C. IR (KBr) νmax: 3043, 2980, 2368, 1934, 1721, 1599, 1522, 1479, 1385, 1317 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.07 (s, 1H), 8.40 (d, J = 8.0 Hz, 2H), 8.19 (d, J = 8.4 Hz, 2H), 7.90 (d, J = 8.8 Hz, 2H), 7.74 (d, J = 9.6 Hz, 1H), 7.56–7.47 (m, 4H), 4.42 (q, J = 7.2 Hz, 2H), 1.44 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.3, 156.4, 144.7, 132.4, 132.3, 131.4, 131.1, 130.9, 130.2, 130.1, 128.8, 127.5, 123.8, 122.0, 117.9, 61.3, 14.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H18ClN4O2: 405.1118; found: 405.1110.
(E)-6-Bromo-2-phenyl-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3da). Orange solid (76%, 59 mg), mp 136–137 °C. IR (KBr) νmax: 3130, 3038, 3001, 2917, 2857, 1933, 1894, 1599, 1474, 1382, 1325, 1237 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.15 (s, 1H), 8.41 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 9.2 Hz, 1H), 7.54–7.46 (m, 4H), 7.32 (d, J = 8.0 Hz, 2H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 151.7, 150.4, 144.3, 140.6, 133.0, 132.3, 132.1, 130.1, 130.0, 129.5, 129.3, 128.7, 122.3, 118.1, 109.5, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16BrN4: 391.0558; found: 391.0552.
(E)-Ethyl-4-((6-cyano-2-phenylimidazo[1,2-a]pyridin-3-yl)diazenyl)benzoate (3ef). Orange solid (88%, 70 mg), mp 184–186 °C. IR (KBr) νmax: 3401, 3120, 3046, 2984, 2234, 1934, 1713, 1599, 1524, 1482, 1387, 1334, 1308 cm−1; 1H NMR (400 MHz, CDCl3): δ 10.35 (s, 1H), 8.40 (d, J = 6.4 Hz, 2H), 8.20 (d, J = 8.4 Hz, 2H), 7.91 (d, J = 8.4 Hz, 2H), 7.82 (d, J = 8.8 Hz, 1H), 7.60–7.53 (m, 4H), 4.43 (d, J = 7.2 Hz, 2H), 1.44 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.1, 156.0, 135.3, 132.0, 131.7, 131.0, 130.9, 130.6, 130.3, 129.8, 128.9, 122.2, 121.8, 118.6, 116.4, 101.5, 61.4, 14.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H18N5O2: 396.1460; found: 396.1452.
(E)-2-(Naphthalen-2-yl)-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3fa). Orange solid (90%, 65 mg), mp 111–113 °C. IR (KBr) νmax: 3080, 3018, 1506, 1487, 1458, 1394, 1385, 1352, 1323, 1302, 1248, 1240 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.97 (s, 1H), 8.97 (s, 1H), 8.60 (d, J = 8.8 Hz, 1H), 8.00–7.96 (m, 2H), 7.90–7.88 (m, 1H), 7.82–7.76 (m, 3H), 7.54–7.45 (m, 3H), 7.31 (d, J = 7.6 Hz, 2H), 7.03 (s, 1H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 152.0, 150.1, 146.1, 140.0, 133.8, 133.5, 132.5, 130.8, 130.1, 130.0, 129.5, 129.4, 129.1, 128.0, 127.8, 127.1, 126.9, 126.3, 122.1, 117.5, 115.2, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H19N4: 363.1610; found: 363.1603.
(E)-2-(3-(p-Tolyldiazenyl)imidazo[1,2-a]pyridin-2-yl)phenol (3ga). Orange solid (82%, 54 mg), mp 120–122 °C. IR (KBr) νmax: 3086, 3024, 2916, 2858, 2806, 2725, 2644, 2557, 1618, 1587, 1578, 1512, 1497, 1408, 1385, 1354, 1323, 1303, 1245, 1234 cm−1; 1H NMR (400 MHz, CDCl3): δ 13.09 (brs, 1H), 10.00 (d, J = 6.4 Hz, 1H), 8.74 (d, J = 7.6 Hz, 1H), 7.88 (d, J = 6.4 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.66 (d, J = 8.8 Hz, 1H), 7.50 (t, J = 7.2 Hz, 1H), 7.38–7.28 (m, 3H), 7.10–6.99 (m, 2H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 159.6, 151.8, 143.6, 140.2, 131.3, 131.2, 130.0, 129.9, 129.5, 122.8, 122.6, 122.1, 119.4, 117.8, 116.5, 116.4, 115.8, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H17N4O: 329.1402; found: 329.1396.
(E)-2-(Thiophen-2-yl)-3-(p-tolyldiazenyl)imidazo[1,2-a]pyridine (3ha). Yellow solid (85%, 54 mg), mp 120–121 °C. IR (KBr) νmax: 3059, 3029, 2917, 2856, 2395, 1952, 1901, 1787, 1716, 1554, 1484, 1431, 1384, 1331 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.86 (d, J = 6.8 Hz, 1H), 8.13–8.12 (m, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 9.2 Hz, 1H), 7.55–7.53 (m, 1H), 7.46–7.42 (m, 1H), 7.31 (d, J = 8.4 Hz, 2H), 7.22–7.20 (m, 1H), 7.00 (t, J = 7.0 Hz, 1H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 152.0, 146.4, 146.1, 140.0, 135.7, 130.9, 130.0, 129.8, 129.6, 129.2, 128.1, 122.3, 117.3, 115.0, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H15N4S: 319.1017; found: 319.1015.
(E)-8-Methyl-2-phenyl-3-(pyridin-3-yldiazenyl)imidazo[1,2-a]pyridine (3am). Orange solid (79%, 49 mg), mp 145–146 °C. IR (KBr) νmax: 3030, 2918, 2849, 1672, 1580, 1526, 1473, 1447, 1423, 1381, 1350, 1306, 1292, 1261, 1248 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.78 (d, J = 6.8 Hz, 1H), 9.08 (s, 1H), 8.56 (d, J = 3.6 Hz, 1H), 8.39 (d, J = 7.2 Hz, 2H), 8.02 (d, J = 8.0 Hz, 1H), 7.52–7.43 (m, 3H), 7.40–7.30 (m, 1H), 7.30 (d, J = 6.8 Hz, 1H), 6.97 (t, J = 6.8 Hz, 1H), 2.70 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 151.8, 149.6, 149.4, 146.7, 146.4, 133.2, 132.9, 130.1, 129.5, 128.6, 127.7, 127.4, 126.1, 124.1, 115.7, 16.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H16N5: 314.1406; found: 314.1401.
(E)-8-Methyl-2-phenyl-3-((3-phenyl-1H-pyrazol-5-yl)diazenyl)imidazo[1,2-a]pyridine (3an). Brown solid (74%, 56 mg), mp 135–137 °C. IR (KBr) νmax: 3142, 3067, 3013, 2880, 1483, 1373, 1308, 1249 cm−1; 1H NMR (500 MHz, DMSO-d6): δ 9.69 (d, J = 6.5 Hz, 1H), 8.41 (d, J = 7.5 Hz, 2H), 7.87 (d, J = 7.5 Hz, 2H), 7.58 (t, J = 7.5 Hz, 2H), 7.50–7.47 (m, 5H), 7.39 (t, J = 7.5 Hz, 1H), 7.21 (t, J = 7.0 Hz, 1H), 6.91 (s, 1H), 2.61 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 148.5, 145.7, 132.7, 132.1, 129.7, 129.6, 129.4, 129.1, 128.9, 128.7, 128.5, 126.9, 125.4, 116.2, 90.8, 16.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H19N6: 379.1671; found: 379.1668.
(E)-2-(p-Tolyl)-3-(p-tolyldiazenyl)benzo[d]imidazo[2,1-b]thiazole (5aa). Yellow solid (92%, 70 mg), mp 128–129 °C. IR (KBr) νmax: 3095, 3058, 3020, 2708, 2272, 1485, 1474, 1356, 1335, 1236 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.14 (d, J = 8.0 Hz, 1H), 8.20 (d, J = 8.4 Hz, 2H), 7.81 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 7.6 Hz, 1H), 7.46 (t, J = 7.2 Hz, 1H), 7.38–7.30 (m, 5H), 2.45 (s, 3H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 153.4, 151.7, 150.9, 140.4, 139.1, 138.8, 134.4, 130.8, 130.1, 130.0, 129.7, 129.3, 126.0, 125.4, 123.5, 122.3, 119.6, 21.63, 21.57; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H19N4S: 383.1330; found: 383.1327.
(E)-3-((2-Bromo-5-(trifluoromethyl)phenyl)diazenyl)-2-phenylbenzo[d]imidazo[2,1-b]thiazole (5bo). Yellow solid (93%, 93 mg), mp 161–162 °C. IR (KBr) νmax: 3086, 3057, 3020, 2930, 1516, 1474, 1423, 1377, 1342, 1317, 1256, 1232 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.51 (d, J = 8.8 Hz, 1H), 8.27 (d, J = 7.6 Hz, 2H), 8.00 (s, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.51–7.41 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 156.1, 155.5, 151.9, 137.6, 134.7, 133.9, 132.9, 130.0 (q, 2JCF = 33.0 Hz), 130.3, 129.9, 129.8, 128.6, 126.9, 126.5, 126.1 (q, 3JCF = 3.8 Hz), 126.0, 123.8 (q, 1JCF = 270.8 Hz), 123.5, 120.4, 116.3 (q, 3JCF = 4.5 Hz); HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H13BrF3N4S: 500.9996; found: 501.0000.
(E)-6-Phenyl-5-(p-tolyldiazenyl)imidazo[2,1-b]thiazole (5ca). Yellow solid (89%, 57 mg), mp 126–127 °C. IR (KBr) νmax: 3138, 3109, 3096, 3061, 3030, 2804, 2249, 1601, 1522, 1475, 1456, 1437, 1369, 1348, 1325, 1294, 1250 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.50 (s, 1H), 8.37 (d, J = 7.6 Hz, 2H), 7.74 (d, J = 7.6 Hz, 2H), 7.50 (t, J = 7.2 Hz, 2H), 7.41 (t, J = 7.2 Hz, 1H), 7.28 (d, J = 8.0 Hz, 2H), 6.88 (d, J = 3.6 Hz, 1H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 154.2, 151.5, 149.6, 140.3, 136.1, 133.4, 129.9, 129.0, 128.9, 128.6, 123.2, 122.3, 113.1, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H15N4S: 319.1017; found: 319.1015.
(E)-Methyl-3-((4-(1-ethoxycarbonyl)phenyl)diazenyl) indolizine-1-carboxylate (7af). Red solid (94%, 66 mg), mp 97–98 °C. IR (KBr) νmax: 3404, 3370, 3125, 3096, 3083, 2979, 2956, 2900, 2866, 2835, 2639, 2327, 2260, 1956, 1911, 1707, 1700, 1599, 1521, 1418, 1388, 1343 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.32 (brs, 1H), 8.27 (d, J = 8.8 Hz, 1H), 8.08 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 7.6 Hz, 3H), 7.34 (t, J = 7.6 Hz, 1H), 6.99 (t, J = 6.8 Hz, 1H); 4.38 (q, J = 7.2 Hz, 2H), 3.89 (s, 3H), 1.41 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.3, 164.7, 156.1, 138.2, 130.6, 130.5, 127.6, 121.7, 119.9, 115.5, 108.3, 61.2, 51.5, 14.4; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H18N3O4: 352.1297; found: 352.1291.
(E)-3-((4-Acetylphenyl)diazenyl)indolizine-1-carbonitrile (7bg). Red solid (92%, 53 mg), mp 143–145 °C. IR (KBr) νmax: 3135, 3107, 2369, 2217, 1934, 1677, 1490, 1422, 1350 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.42 (brs, 1H), 8.06 (d, J = 8.0 Hz, 2H), 7.89 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 8.0 Hz, 1H), 7.65 (s, 1H), 7.44 (t, J = 6.8 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 2.65 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 197.4, 156.0, 139.6, 137.6, 129.6, 127.6, 126.3, 126.21, 126.15, 122.3, 118.1, 116.1, 115.3, 87.2, 26.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H13N4O: 289.1089; found: 289.1086.
Benzo[e]pyrrolo[2,1-c][1,2,4]triazine (9). Yellow solid (78%, 26 mg), mp 176–178 °C. IR (KBr) νmax: 3119, 3109, 3055, 3020, 1792, 1706, 1610, 1578, 1524, 1506, 1491, 1456, 1406, 1356, 1308, 1277, 1236 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.42 (d, J = 8.4 Hz, 1H), 7.85–7.83 (m, 2H), 7.72 (t, J = 7.6 Hz, 1H), 7.57 (t, J = 8.0 Hz, 1H), 7.42 (d, J = 4.0 Hz, 1H), 7.10 (t, J = 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 141.3, 136.5, 132.3, 130.8, 125.9, 124.9, 116.4, 113.5, 111.0, 109.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C10H8N3: 170.0718; found: 170.0715.
Acknowledgements
A. H. acknowledges the financial support from DST, GoWB (ST/P/S&T/4G-2/2014). A. C. is thankful to UGC (Dr D. S. Kothari Post Doctoral Fellowship) and A. D. is thankful to CSIR for their fellowships.
Notes and references
-
(a) R. P. Pandit and Y. R. Lee, Adv. Synth. Catal., 2015, 357, 2657 CrossRef CAS;
(b) M. A. Metwally, E. Abdel-Galil, A. Metwally and F. A. Amer, Dyes Pigm., 2012, 92, 902 CrossRef CAS;
(c) A. D. Towns, Dyes Pigm., 1999, 42, 3 CrossRef CAS;
(d) V. Kumar, K. Kaur, G. K. Gupta and A. K. Sharma, Eur. J. Med. Chem., 2013, 69, 735 CrossRef CAS PubMed;
(e) Y. Deng, B. Tang, H. Zhao, J. Xu, J. Xiao, X. Zhang, H. Xu and S. Zhang, Color. Technol., 2012, 129, 144 CrossRef.
-
(a) T. Wendler, C. Schütt, C. Naäther and R. Herges, J. Org. Chem., 2012, 77, 3284 CrossRef CAS PubMed;
(b) S. Thies, H. Sell, C. Schuütt, C. Bornholdt, C. Naäther, F. Tuczek and R. Herges, J. Am. Chem. Soc., 2011, 133, 16243 CrossRef CAS PubMed;
(c) Y. Chen, H. Yu, M. Quan, L. Zhang, H. Yang and Y. Lu, RSC Adv., 2015, 5, 4675 RSC.
-
(a) A. C. Razus, L. Birzan, N. M. Surugiu, A. C. Corbu and F. Chiraleu, Dyes Pigm., 2007, 74, 26 CrossRef CAS;
(b) C. R. Moylan, R. J. Twieg, V. Y. Lee, S. A. Swanson, K. M. Betterton and R. D. Miller, J. Am. Chem. Soc., 1993, 115, 12599 CrossRef CAS.
- J. Belmar, M. Parra, C. Zúñiga, C. Pérez and C. Muñoz, Liq. Cryst., 1999, 26, 389 CrossRef CAS.
- M. A. Salvador, L. V. Reis, P. Almeida and P. F. Santos, Tetrahedron, 2008, 64, 209 CrossRef.
-
(a) S. Okumura, C.-H. Lin, Y. Takeda and S. Minakata, J. Org. Chem., 2013, 78, 12090 CrossRef CAS PubMed;
(b) D. Anand, O. P. S. Patel, R. K. Maurya, R. Kant and P. P. Yadav, J. Org. Chem., 2015, 80, 12410 CrossRef CAS PubMed;
(c) R. K. Khalifa, M. A. Metwally, E. Abdel-latif and F. A. Amer, J. Text. Sci. Eng., 2012, 1, 1 Search PubMed;
(d) B. F. Abdel-Wahab, A.-A. El-Ahl and F. A. Badria, Chem. Pharm. Bull., 2009, 57, 1348 CrossRef CAS PubMed;
(e) A. Sharma, B. K. Sarangi and R. K. Behera, J. Indian Chem. Soc., 1985, 62, 253 CAS;
(f) A. S. Shawali, M. Sami and S. M. Sherif, J. Heterocycl. Chem., 1980, 17, 877 CrossRef CAS;
(g) H. M. Hassaneen, A. S. Shawali, N. M. Elwan and N. M. Abounada, Org. Prep. Proced. Int., 1992, 24, 171 CrossRef CAS;
(h) H. Faustino, R. M. El-Shishtawy, L. V. Reis, P. F. Santos and P. Almeida, Tetrahedron Lett., 2008, 49, 6907 CrossRef CAS.
-
(a) C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS PubMed;
(b) A. T. Baviskar, S. M. Amrutkar, N. Trivedi, V. Chaudhary, A. Nayak, S. K. Guchhait, U. C. Banerjee, P. V. Bharatam and C. N. Kundu, ACS Med. Chem. Lett., 2015, 6, 481 CrossRef CAS PubMed.
-
(a) A. J. Stasyuk, M. Banasiewicz, M. K. Cyrański and D. T. Gryko, J. Org. Chem., 2012, 77, 5552 CrossRef CAS PubMed;
(b) N. Shao, G.-X. Pang, C.-X. Yan, G.-F. Shi and Y. Cheng, J. Org. Chem., 2011, 76, 7458 CrossRef CAS PubMed.
-
(a) K. Pericherla, P. Kaswan, K. Pandey and A. Kumar, Synthesis, 2015, 47, 887 CrossRef CAS;
(b) A. K. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. Commun., 2015, 51, 1555 RSC;
(c) H. Cao, X. Liu, J. Liao, J. Huang, H. Qiu, Q. Chen and Y. Chen, J. Org. Chem., 2014, 79, 11209 CrossRef CAS PubMed;
(d) H. Cao, S. Lei, N. Li, L. Chen, J. Liu, H. Cai, S. Qiu and J. Tan, Chem. Commun., 2015, 51, 1823 RSC.
-
(a) S. Mishra, K. Monir, S. Mitra and A. Hajra, Org. Lett., 2014, 16, 6084 CrossRef CAS PubMed;
(b) K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, Org. Lett., 2014, 16, 4630 CrossRef CAS PubMed;
(c) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef CAS;
(d) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS.
-
(a) K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, J. Org. Chem., 2015, 80, 1332 CrossRef CAS PubMed;
(b) S. Mitra, M. Ghosh, S. Mishra and A. Hajra, J. Org. Chem., 2015, 80, 8275 CrossRef CAS PubMed;
(c) K. Monir, M. Ghosh, S. Jana, P. Mondal, A. Majee and A. Hajra, Org. Biomol. Chem., 2015, 13, 8717 RSC.
- CCDC 1420103.†.
- H. GroB and J. Gloede, Angew. Chem., 1963, 75, 376 Search PubMed.
- H. Xu and L.-I. Fan, Eur. J. Med. Chem., 2011, 46, 364 CrossRef CAS PubMed.
-
(a) K. Barral, A. D. Moorhouse and J. E. Moses, Org. Lett., 2007, 9, 1809 CrossRef CAS PubMed;
(b) D. Qiu, H. Meng, L. Jin, S. Wang, S. Tang, X. Wang, F. Mo, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2013, 52, 11581 CrossRef CAS PubMed.
- L. Zhang, F. Liang, L. Sun, Y. Hu and H. Hu, Synthesis, 2000, 12, 1733 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1420103. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra03070j |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 









