Design, synthesis and evaluation of benzenesulfonamide-substituted 1,5-diarylpyrazoles containing phenylacetohydrazide derivatives as COX-1/COX-2 agents against solid tumors†
Abstract
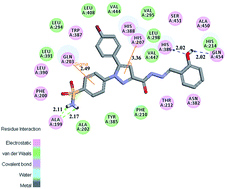
Novel benzenesulfonamide-substituted 1,5-diarylpyrazoles containing phenylacetohydrazide derivatives have been designed, synthesized and evaluated for their biological activities as selective COX-2 inhibitors with anticancer potential. In vitro the bioassay results revealed that some of them displayed potent inhibitory activity in the enzymatic and cellular assays. Among them, compound 48 showed the most powerful and potent selective inhibitory activity (IC50 = 82.21 μM for COX-1 and IC50 = 0.37 μM for COX-2), comparable to the control positive compound Celecoxib (40.29 μM, 0.15 μM). Antiproliferative assay results indicated that compound 48 possess potent antiproliferative activity against A549 cells in vitro with an IC50 value of 0.78 μM. We then performed a PI staining assay and cell apoptosis analysis for compound 48 and found that it effectively causes A549 cell apoptosis. A docking simulation was further performed to position compound 48 into the COX-2 active site to determine the probable binding model. The 3D-QSAR models were built for reasonable design of selective COX-2 inhibitors in the future.

 Please wait while we load your content...
Please wait while we load your content...