Role of functionalization: strategies to explore potential nano-bio applications of magnetic nanoparticles
Abstract
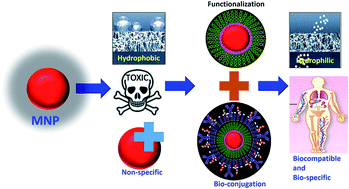
Advances in nanotechnology enable the production of magnetic nanoparticles (MNPs) with specific morphologies and the tailoring of their surfaces in order to manipulate their characteristics to suit specific applications. Magnetic nanoparticles are well-established nanomaterials that offer controlled size, biospecificity, and the ability to be manipulated externally. As a result, these nanoparticles can be explored with respect to a wider range of applications. It is essential to ensure the proper and specific functionalization of magnetic nanoparticles for their successful use in nano-bio applications. The current review focuses on the theoretical background regarding the functionalization of magnetic nanoparticles for nano-bio applications. The review covers all the important aspects regarding the functionalization of magnetic nanoparticles. The ideal requirements for their functionalization for nano-bio applications and the mechanisms behind this are explained. Different materials used in the functionalization of magnetic nanoparticles are classified and discussed in detail. The principle criteria for bioconjugation and different bioconjugation strategies are overviewed. Functionalization strategies for magnetic nanoparticles for specific bioapplications, such as magnetic hyperthermia, magnetic resonance imaging, target drug delivery, bacteria detection, enzyme immobilization, cell labelling, magnetic separation, and DNA enrichment, are highlighted.

 Please wait while we load your content...
Please wait while we load your content...