Facile synthesis of highly porous N-doped CNTs/Fe3C and its electrochemical properties†
Abstract
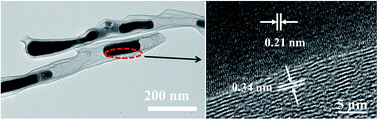
Highly porous N-doped CNTs/Fe3C was synthesized by a facile and one-pot method. In this method, melamine was used as the carbon precursor; iron chloride was employed as a catalyst of graphic carbon and iron carbon source, and zinc chloride as a chemical activator to obtain highly porous structure. Fe3C nanorods were in situ formed and embedded into the inner structure of CNTs. The pore structure analysis shows that N-doped CNTs/Fe3C possesses the large specific surface area up to 1021.26 m2 g−1. The N-doped CNTs/Fe3C electrode exhibits a high specific capacitance of 181 F g−1 at the current density of 0.1 A g−1, and excellent capacitance rate and cycling stability.

 Please wait while we load your content...
Please wait while we load your content...