Solvothermally synthesized SnS nanorods with high carrier mobility leading to thermoelectric enhancement†
Abstract
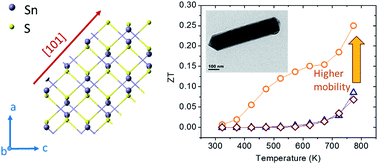
Rod-shaped SnS nanocrystals were synthesized by a facile solvothermal method, which were then compacted by a cold isostatic pressing and rapid annealing process. Two-order higher carrier mobility was achieved in the corresponding bulk samples, leading to over 200% enhancement of thermoelectric figure of merit.

 Please wait while we load your content...
Please wait while we load your content...