Au1−xCux colloidal nanoparticles synthesized via a one-pot approach: understanding the temperature effect on the Au : Cu ratio†
Abstract
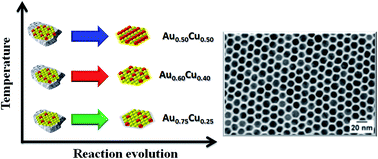
Gold–copper nanoparticles (Au1−xCux NPs) have been reported as a versatile system to study various aspects related to the colloidal synthesis of bimetallic nanoparticles. In this work, Au1−xCux NPs are synthesized via a one pot approach using equimolar amounts of Au and Cu. Different compositions are obtained by changing the reaction temperature. By taking aliquots at crucial stages of the synthesis and combining several techniques, such as elemental analysis, X-ray diffraction and X-ray photoelectron spectroscopy, it was possible to shed light on the synthesis mechanism: the formation of Au NPs occurs at low temperature, then the higher the synthesis temperature the higher the Cu content in the NPs. In parallel to the Au NPs formation, results of energy-dispersive X-ray spectroscopy analyses carried out in scanning transmission electron microscopy mode suggest the formation of a Cu-rich phase at the early stages of the synthesis. Such a phase, not detectable by X-ray diffraction, acts as a reservoir of Cu species that are slowly released to form the Au1−xCux alloy NPs through a digestive ripening-like process. The reaction temperature and annealing time affect the final Au : Cu ratio but had no significant effect on the final size of the particles, which for all compositions is approximately 14 nm.

 Please wait while we load your content...
Please wait while we load your content...