A novel phthalonitrile monomer with low post cure temperature and short cure time
Abstract
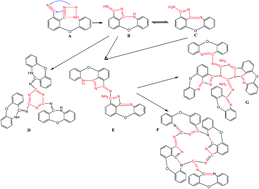
To depress the post cure temperature and time, 3-(2-aminophenoxy)-phthalonitrile (3-APN) was designed and synthesized via the nucleophilic displacement reaction of 3-nitrophthalonitrile and o-aminophenol. The structure of the desired product (3-APN) was confirmed by H nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FTIR). The polymers from the monomer were also characterized by FTIR. The cure behavior of the monomer was investigated by differential scanning calorimetry (DSC). Cure characteristic temperatures were obtained through an extrapolation method. The complex viscosity of the melt was measured by rheometric analysis. The results show that there is no residual and shift absorption of nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) in the infrared spectrogram of the polymers from 3-APN. Viscosity measurement shows that the monomer exhibits a low complex melt viscosity (0.01–0.1 Pa S) in a wide temperature range (145–250 °C). The DSC results show that the polymerization enthalpy of the monomer is 414 J g−1, which is 3 times higher than that of the reported auto-catalytic monomers. Thermo-gravimetric (TG) results of the derived polymers under different cure procedures indicate that when this novel monomer is cured at 250–280 °C for 0.5–1.0 hours, the thermal stability of the derived polymer is equal to or better than most of the reported PN resins which are cured at 370–420 °C for more than 14 hours. And when the curing temperature is gradually increased to 360 °C and the total cure time is only 2 hours, the cured polymer has even more excellent thermal stability with the T5% up to 518 °C and char yield at 800 °C up to 77%.
N) in the infrared spectrogram of the polymers from 3-APN. Viscosity measurement shows that the monomer exhibits a low complex melt viscosity (0.01–0.1 Pa S) in a wide temperature range (145–250 °C). The DSC results show that the polymerization enthalpy of the monomer is 414 J g−1, which is 3 times higher than that of the reported auto-catalytic monomers. Thermo-gravimetric (TG) results of the derived polymers under different cure procedures indicate that when this novel monomer is cured at 250–280 °C for 0.5–1.0 hours, the thermal stability of the derived polymer is equal to or better than most of the reported PN resins which are cured at 370–420 °C for more than 14 hours. And when the curing temperature is gradually increased to 360 °C and the total cure time is only 2 hours, the cured polymer has even more excellent thermal stability with the T5% up to 518 °C and char yield at 800 °C up to 77%.

 Please wait while we load your content...
Please wait while we load your content...